Effect of NaCl and Na2SO4 on Low Temperature Corrosion of Vapour- and Pack-Aluminide Coated Single Crystal Turbine Blade Alloys CMSX-4 and RR3010
- PMID: 37360909
- PMCID: PMC10243695
- DOI: 10.1007/s11661-023-07099-5
Effect of NaCl and Na2SO4 on Low Temperature Corrosion of Vapour- and Pack-Aluminide Coated Single Crystal Turbine Blade Alloys CMSX-4 and RR3010
Abstract
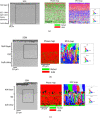
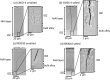
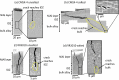
The current work presents a systematic study of two alloy compositions (RR3010 and CMSX-4) and two types of coatings: inward grown (pack) and outward grown (vapour) deposited aluminides, exposed to 98Na2SO4-2NaCl mixture. Grit blasting was used on some of the samples, prior to coating, to mimic in-service procedures and remove oxides from the surface prior to coating. Two-point bend tests were then performed on the coated samples, with and without applied salt at 550 °C for 100 hours. Samples were pre-strained at 0.6 pct strain to deliberately pre-crack the coating and then strained at 0.3 pct for the heat treatment. Exposure to 98Na2SO4-2NaCl under applied stress of vapour-aluminide coated samples of both alloys, revealed significant coating damage in the form of secondary cracks in the intermetallic-rich inter-diffusion zone, although only CMSX-4 exhibited cracks propagating further into the bulk alloy while RR3010 proved more resistant. The pack-aluminide coating proved more protective for both alloys, with cracks propagating only into the coating and never into the underlying alloy. In addition, grit blasting proved beneficial in reducing spallation and cracking for both types of coating. The findings were used to propose a mechanism based on thermodynamic reactions, to explain the crack width changes through the formation of volatile AlCl3 in the cracks.
© The Author(s) 2023.
Conflict of interest statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Figures








References
-
- Reed RC. The Superalloys: Fundamentals and Applications. 1. Cambridge: Cambridge University Press; 2006.
-
- Erikson GL. Superalloys. 1996;1996:36.
-
- Wahl J, Harris K. Proc. ASME Turbo Expo. 2020;8:1–10.
-
- S. Tin, L. Zhang, R.A. Hobbs, A.-C. Yeh, C.M.F. Rae, and B. Broomfield: in Superalloys 2008 (Eleventh International Symposium), TMS, 2008, pp. 81–90.
-
- Segersäll M, Kontis P, Pedrazzini S, Bagot PAJ, Moody MP, Moverare JJ, Reed RC. Acta Mater. 2015 doi: 10.1016/j.actamat.2015.03.060. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
