Metformin alleviates lung-endothelial hyperpermeability by regulating cofilin-1/PP2AC pathway
- PMID: 37361221
- PMCID: PMC10285707
- DOI: 10.3389/fphar.2023.1211460
Metformin alleviates lung-endothelial hyperpermeability by regulating cofilin-1/PP2AC pathway
Abstract
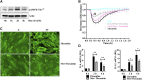
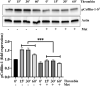
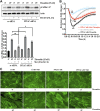
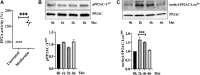
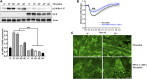
Background: Microvascular endothelial hyperpermeability is an earliest pathological hallmark in Acute Lung Injury (ALI), which progressively leads to Acute Respiratory Distress Syndrome (ARDS). Recently, vascular protective and anti-inflammatory effect of metformin, irrespective of glycemic control, has garnered significant interest. However, the underlying molecular mechanism(s) of metformin's barrier protective benefits in lung-endothelial cells (ECs) has not been clearly elucidated. Many vascular permeability-increasing agents weakened adherens junctions (AJ) integrity by inducing the reorganization of the actin cytoskeleton and stress fibers formation. Here, we hypothesized that metformin abrogated endothelial hyperpermeability and strengthen AJ integrity via inhibiting stress fibers formation through cofilin-1-PP2AC pathway. Methods: We pretreated human lung microvascular ECs (human-lung-ECs) with metformin and then challenged with thrombin. To investigate the vascular protective effects of metformin, we studied changes in ECs barrier function using electric cell-substrate impedance sensing, levels of actin stress fibers formation and inflammatory cytokines IL-1β and IL-6 expression. To explore the downstream mechanism, we studied the Ser3-phosphorylation-cofilin-1 levels in scramble and PP2AC-siRNA depleted ECs in response to thrombin with and without metformin pretreatment. Results: In-vitro analyses showed that metformin pretreatment attenuated thrombin-induced hyperpermeability, stress fibers formation, and the levels of inflammatory cytokines IL-6 and IL-β in human-lung-ECs. We found that metformin mitigated Ser3-phosphorylation mediated inhibition of cofilin-1 in response to thrombin. Furthermore, genetic deletion of PP2AC subunit significantly inhibited metformin efficacy to mitigate thrombin-induced Ser3-phosphorylation cofilin-1, AJ disruption and stress fibers formation. We further demonstrated that metformin increases PP2AC activity by upregulating PP2AC-Leu309 methylation in human-lung-ECs. We also found that the ectopic expression of PP2AC dampened thrombin-induced Ser3-phosphorylation-mediated inhibition of cofilin-1, stress fibers formation and endothelial hyperpermeability. Conclusion: Together, these data reveal the unprecedented endothelial cofilin-1/PP2AC signaling axis downstream of metformin in protecting against lung vascular endothelial injury and inflammation. Therefore, pharmacologically enhancing endothelial PP2AC activity may lead to the development of novel therapeutic approaches for prevention of deleterious effects of ALI on vascular ECs.
Keywords: PP2A; acute lung injury; cofilin-1; metformin; vascular endothelial cells.
Copyright © 2023 Siddiqui, Reddy, Faridi, Shahid and Shanley.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Ambach A., Saunus J., Konstandin M., Wesselborg S., Meuer S. C., Samstag Y. (2000). The serine phosphatases PP1 and PP2A associate with and activate the actin-binding protein cofilin in human T lymphocytes. Eur. J. Immunol. 30 (12), 3422–3431. 10.1002/1521-4141(2000012)30:12<3422:AID-IMMU3422>3.0.CO;2-J - DOI - PubMed
LinkOut - more resources
Full Text Sources

