HIF and MYC signaling in adrenal neoplasms of the neural crest: implications for pediatrics
- PMID: 37361539
- PMCID: PMC10286580
- DOI: 10.3389/fendo.2023.1022192
HIF and MYC signaling in adrenal neoplasms of the neural crest: implications for pediatrics
Abstract
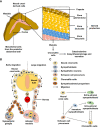
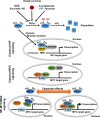
Pediatric neural crest-derived adrenal neoplasms include neuroblastoma and pheochromocytoma. Both entities are associated with a high degree of clinical heterogeneity, varying from spontaneous regression to malignant disease with poor outcome. Increased expression and stabilization of HIF2α appears to contribute to a more aggressive and undifferentiated phenotype in both adrenal neoplasms, whereas MYCN amplification is a valuable prognostic marker in neuroblastoma. The present review focuses on HIF- and MYC signaling in both neoplasms and discusses the interaction of associated pathways during neural crest and adrenal development as well as potential consequences on tumorigenesis. Emerging single-cell methods together with epigenetic and transcriptomic analyses provide further insights into the importance of a tight regulation of HIF and MYC signaling pathways during adrenal development and tumorigenesis. In this context, increased attention to HIF-MYC/MAX interactions may also provide new therapeutic options for these pediatric adrenal neoplasms.
Keywords: MYC; catecholamines; hypoxia; neural crest; neuroblastoma; paraganglioma; pheochromocytoma; sympathoadrenal cell lineage.
Copyright © 2023 Bechmann, Westermann and Eisenhofer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Neural crest development and neuroblastoma: the genetic and biological link.Prog Brain Res. 2004;146:233-42. doi: 10.1016/s0079-6123(03)46015-9. Prog Brain Res. 2004. PMID: 14699967
-
CD44-high neural crest stem-like cells are associated with tumour aggressiveness and poor survival in neuroblastoma tumours.EBioMedicine. 2019 Nov;49:82-95. doi: 10.1016/j.ebiom.2019.10.041. Epub 2019 Nov 2. EBioMedicine. 2019. PMID: 31685444 Free PMC article.
-
High levels of HIF-2alpha highlight an immature neural crest-like neuroblastoma cell cohort located in a perivascular niche.J Pathol. 2008 Mar;214(4):482-8. doi: 10.1002/path.2304. J Pathol. 2008. PMID: 18189331
-
CD114: A New Member of the Neural Crest-Derived Cancer Stem Cell Marker Family.J Cell Biochem. 2017 Feb;118(2):221-231. doi: 10.1002/jcb.25656. Epub 2016 Aug 16. J Cell Biochem. 2017. PMID: 27428599 Review.
-
The MYCN oncogene and differentiation in neuroblastoma.Semin Cancer Biol. 2011 Oct;21(4):256-66. doi: 10.1016/j.semcancer.2011.08.001. Epub 2011 Aug 9. Semin Cancer Biol. 2011. PMID: 21849159 Review.
Cited by
-
Molecular Genetics of Pheochromocytoma/Paraganglioma.Curr Opin Endocr Metab Res. 2024 Sep;36:100527. doi: 10.1016/j.coemr.2024.100527. Epub 2024 May 31. Curr Opin Endocr Metab Res. 2024. PMID: 39328362
-
Adrenocorticotropin-Secreting Pure Adrenal Ganglioneuroma Leading to Cushing Syndrome.JCEM Case Rep. 2025 Feb 10;3(3):luaf027. doi: 10.1210/jcemcr/luaf027. eCollection 2025 Mar. JCEM Case Rep. 2025. PMID: 39935496 Free PMC article.
-
Genotype and clinical phenotype characteristics of MAX germline mutation-associated pheochromocytoma/paraganglioma syndrome.Front Endocrinol (Lausanne). 2024 Aug 30;15:1442691. doi: 10.3389/fendo.2024.1442691. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39279998 Free PMC article.
-
Target Genes of c-MYC and MYCN with Prognostic Power in Neuroblastoma Exhibit Different Expressions during Sympathoadrenal Development.Cancers (Basel). 2023 Sep 16;15(18):4599. doi: 10.3390/cancers15184599. Cancers (Basel). 2023. PMID: 37760568 Free PMC article.
-
Molecular principles underlying aggressive cancers.Signal Transduct Target Ther. 2025 Feb 17;10(1):42. doi: 10.1038/s41392-025-02129-7. Signal Transduct Target Ther. 2025. PMID: 39956859 Free PMC article. Review.
References
-
- Holmquist-Mengelbier L, Fredlund E, Löfstedt T, Noguera R, Navarro S, Nilsson H, et al. . Recruitment of HIF-1α and HIF-2α to common target genes is differentially regulated in neuroblastoma: HIF-2α promotes an aggressive phenotype. Cancer Cell (2006) 10:413–23. doi: 10.1016/j.ccr.2006.08.026 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

