Factors associated with shorter-interval cervical cancer screening for young women in three United States healthcare systems
- PMID: 37361923
- PMCID: PMC10285268
- DOI: 10.1016/j.pmedr.2023.102279
Factors associated with shorter-interval cervical cancer screening for young women in three United States healthcare systems
Abstract
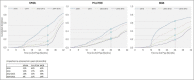
Frequently changing cervical cancer screening guidelines over the past two decades have been inconsistently adopted in the United States. Current guidelines set the recommended screening interval to three years for average-risk women aged 21-29 years. Few studies have evaluated how patient and provider factors are associated with implementation of cervical cancer screening intervals among younger women. This study evaluated multilevel factors associated with screening interval length among 69,939 women aged 21-29 years with an initial negative Pap screen between 2010 and 2015 across three large health systems in the U.S. Shorter-interval screening was defined as a second screening Pap within 2.5 years of an initial negative Pap. Mixed-effects logistic regression was performed for each site to identify provider and patient characteristics associated with shorter-interval screening. The odds of shorter-interval screening decreased over the study period across all sites, though the proportion of patients screened within 2.5 years remained between 7.5% and 20.7% across sites in 2014-2015. Patient factors including insurance, race/ethnicity, and pregnancy were associated with shorter-interval screening, though the patterns differed across sites. At one site, the variation in shorter-interval screening explained by the provider was 10.6%, whereas at the other two sites, the provider accounted for < 2% of the variation in shorter-interval screening. Our results highlight the heterogeneity in factors driving cervical cancer screening interval across health systems and point to the need for tailored approaches targeted to both providers and patients to improve guideline-concordant screening.
Keywords: Cancer prevention; Cervical cancer; Multilevel; Pap screening; Screening interval.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- ACOG Practice Bulletin: Cervical cytology screening. Obstet Gynecol. 2009;114:1409–1420. - PubMed
-
- Updated Cervical Cancer Screening Guidelines Practice Advisory April 2021 American College of Obstetricians and Gynecologists https://www.acog.org/clinical/clinical-guidance/practice-advisory/articl....
-
- Castle P.E., Kinney W.K., Chen L.u., Kim J.J., Jenison S., Rossi G., Kang H., Cuzick J., Wheeler C.M., Joste N.E.W., Kinney C.M., Wheeler C.L., Wiggins M., Robertson R.M., McDonald A., Waxman S., Jenison J., Howe D., Saslow J.J., Kim M.H., Stoler J., Cuzick P.E., Castle R.B., Perkins J.L., Gonzales S., Torres G., Rossi K., English Adherence to National Guidelines on Cervical Screening: A Population-Based Evaluation from a Statewide Registry. J. Natl. Cancer Inst. 2022;114(4):626–630. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous