A versatile, bioengineered skin reconstruction device designed for use in austere environments
- PMID: 37362212
- PMCID: PMC10285514
- DOI: 10.3389/fbioe.2023.1208322
A versatile, bioengineered skin reconstruction device designed for use in austere environments
Abstract
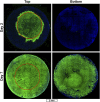
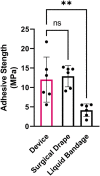
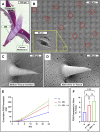
Austere environments in which access to medical facilities, medical personnel, or even water and electricity is limited or unavailable pose unique challenges for medical device product design. Currently existing skin substitutes are severely inadequate for the treatment of severe burns, chronic wounds, battlefield injuries, or work-related injuries in resource-limited settings. For such settings, an ideal device should be biocompatible, bioresorbable, promote tissue healing, not require trained medical personnel for deployment and use, and should enable topical drug delivery. As proof of concept for such a device, silk fibroin and an antioxidant hyaluronic acid derivative were chosen as primary constituents. The final formulation was selected to optimize tensile strength while retaining mechanical compliance and protection from reactive oxygen species (ROS). The ultimate tensile strength of the device was 438.0 KPa. Viability of dermal fibroblasts challenged with ROS-generating menadione decreased to 49.7% of control, which was rescued by pre-treatment with the hyaluronic acid derivative to 85.0% of control. The final device formulation was also tested in a standardized, validated, in vitro skin irritation test which revealed no tissue damage or statistical difference from control. Improved topical drug delivery was achieved via an integrated silk fibroin microneedle array and selective device processing to generate crosslinked/through pores. The final device including these features showed a 223% increase in small molecule epidermal permeation relative to the control. Scaffold porosity and microneedle integrity before and after application were confirmed by electron microscopy. Next, the device was designed to be self-adherent to enable deployment without the need of traditional fixation methods. Device tissue adhesive strength (12.0 MPa) was evaluated and shown to be comparable to a commercial adhesive surgical drape (12.9 MPa) and superior to an over-the-counter liquid bandage (4.1 MPa). Finally, the device's wound healing potential was assessed in an in vitro full-thickness skin wound model which showed promising device integration into the tissue and cellular migration into and above the device. Overall, these results suggest that this prototype, specifically designed for use in austere environments, is mechanically robust, is cytocompatible, protects from ROS damage, is self-adherent without traditional fixation methods, and promotes tissue repair.
Keywords: austere environment; biomaterial; hyaluronic acid; microneedles; silk fibroin; skin reconstruction device; wound healing.
Copyright © 2023 Veit, Weidow and Serban.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Silk Fibroin-Based Bioengineered Scaffold for Enabling Hemostasis and Skin Regeneration of Critical-Size Full-Thickness Heat-Induced Burn Wounds.ACS Biomater Sci Eng. 2022 Sep 12;8(9):3856-3870. doi: 10.1021/acsbiomaterials.2c00328. Epub 2022 Aug 15. ACS Biomater Sci Eng. 2022. PMID: 35969223
-
A digital light processing 3D-printed artificial skin model and full-thickness wound models using silk fibroin bioink.Acta Biomater. 2023 Jul 1;164:159-174. doi: 10.1016/j.actbio.2023.04.034. Epub 2023 Apr 28. Acta Biomater. 2023. PMID: 37121370
-
Silk fibroin for skin injury repair: Where do things stand?Adv Drug Deliv Rev. 2020 Jan 1;153:28-53. doi: 10.1016/j.addr.2019.09.003. Epub 2019 Oct 31. Adv Drug Deliv Rev. 2020. PMID: 31678360 Review.
-
Silk Fibroin Biomaterials and Their Beneficial Role in Skin Wound Healing.Biomolecules. 2022 Dec 12;12(12):1852. doi: 10.3390/biom12121852. Biomolecules. 2022. PMID: 36551280 Free PMC article. Review.
-
In Situ Forming Injectable Silk Fibroin Hydrogel Promotes Skin Regeneration in Full Thickness Burn Wounds.Adv Healthc Mater. 2018 Dec;7(24):e1801092. doi: 10.1002/adhm.201801092. Epub 2018 Oct 31. Adv Healthc Mater. 2018. PMID: 30379407
Cited by
-
Hyaluronic acid-ibuprofen conjugation: a novel ototherapeutic approach protecting inner ear cells from inflammation-mediated damage.Front Pharmacol. 2024 Feb 15;15:1355283. doi: 10.3389/fphar.2024.1355283. eCollection 2024. Front Pharmacol. 2024. PMID: 38425644 Free PMC article.
-
In vitro characterization of novel hyaluronan-antioxidant conjugates as potential topical therapeutics against hearing loss.Front Pharmacol. 2024 Feb 28;15:1355279. doi: 10.3389/fphar.2024.1355279. eCollection 2024. Front Pharmacol. 2024. PMID: 38482050 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

