Fast strain mapping in abdominal aortic aneurysm wall reveals heterogeneous patterns
- PMID: 37362444
- PMCID: PMC10285457
- DOI: 10.3389/fphys.2023.1163204
Fast strain mapping in abdominal aortic aneurysm wall reveals heterogeneous patterns
Abstract
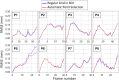
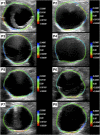
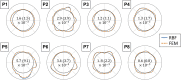
Abdominal aortic aneurysm patients are regularly monitored to assess aneurysm development and risk of rupture. A preventive surgical procedure is recommended when the maximum aortic antero-posterior diameter, periodically assessed on two-dimensional abdominal ultrasound scans, reaches 5.5 mm. Although the maximum diameter criterion has limited ability to predict aneurysm rupture, no clinically relevant tool that could complement the current guidelines has emerged so far. In vivo cyclic strains in the aneurysm wall are related to the wall response to blood pressure pulse, and therefore, they can be linked to wall mechanical properties, which in turn contribute to determining the risk of rupture. This work aimed to enable biomechanical estimations in the aneurysm wall by providing a fast and semi-automatic method to post-process dynamic clinical ultrasound sequences and by mapping the cross-sectional strains on the B-mode image. Specifically, the Sparse Demons algorithm was employed to track the wall motion throughout multiple cardiac cycles. Then, the cyclic strains were mapped by means of radial basis function interpolation and differentiation. We applied our method to two-dimensional sequences from eight patients. The automatic part of the analysis took under 1.5 min per cardiac cycle. The tracking method was validated against simulated ultrasound sequences, and a maximum root mean square error of 0.22 mm was found. The strain was calculated both with our method and with the established finite-element method, and a very good agreement was found, with mean differences of one order of magnitude smaller than the image spatial resolution. Most patients exhibited a strain pattern that suggests interaction with the spine. To conclude, our method is a promising tool for investigating abdominal aortic aneurysm wall biomechanics as it can provide a fast and accurate measurement of the cyclic wall strains from clinical ultrasound sequences.
Keywords: abdominal aortic aneurysm; finite element modeling; radial basis functions; strain imaging; ultrasound B-mode cine-loops; ultrasound elastography; ultrasound simulations; vascular wall strains.
Copyright © 2023 Bracco, Broda, Lorenzen, Florkow, Somphone, Avril, Biancolini and Rouet.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures









References
-
- Alessandrini M., Chakraborty B., Heyde B., Bernard O., De Craene M., Sermesant M., et al. (2018). Realistic vendor-specific synthetic ultrasound data for quality assurance of 2-D speckle tracking echocardiography: Simulation pipeline and open access database. IEEE Trans. Ultrasonics, Ferroelectr. Freq. Control 65, 411–422. 10.1109/TUFFC.2017.2786300 - DOI - PubMed
-
- Alessandrini M., De Craene M., Bernard O., Giffard-Roisin S., Allain P., Waechter-Stehle I., et al. (2015). A pipeline for the generation of realistic 3D synthetic echocardiographic sequences: Methodology and open-access database. IEEE Trans. Med. Imaging 34, 1436–1451. 10.1109/TMI.2015.2396632 - DOI - PubMed
-
- Alessandrini M., Heyde B., Queirós S., Cygan S., Zontak M., Somphone O., et al. (2016). Detailed evaluation of five 3D speckle tracking algorithms using synthetic echocardiographic recordings; detailed evaluation of five 3D speckle tracking algorithms using synthetic echocardiographic recordings. IEEE Trans. Med. Imaging 35. 10.1109/TMI.2016.2537848 - DOI - PubMed
-
- Barlow J. (1976). Optimal stress locations in finite element models. Int. J. Numer. Methods Eng. 10, 243–251.
-
- Biancolini M. E. (2017). Fast radial basis functions for engineering applications. Springer.
LinkOut - more resources
Full Text Sources

