Impact of implementing a category 1 external quality assurance scheme for monitoring harmonization of clinical laboratories in Spain
- PMID: 37362553
- PMCID: PMC10197279
- DOI: 10.1515/almed-2020-0008
Impact of implementing a category 1 external quality assurance scheme for monitoring harmonization of clinical laboratories in Spain
Abstract
Background: The objective of the present study was to examine the evolution of the analytical performance specifications (APS) used in External Quality Assurance (EQA) schemes, as well as the efficacy of a category 1 EQA scheme in monitoring the harmonization of clinical laboratory results in Spain.
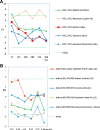
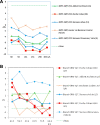
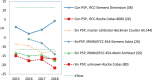
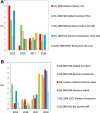
Methods: A review of the literature on the types of quality specifications used in schemes in other countries and their evolution was performed. In addition, a comparative analysis of the potential impact that different APS from eight countries had on clinical decision-making was made based on three measurands: sodium, thyroid-stimulating hormone (TSH), and activated partial thromboplastin time (aPTT).
Results: Harmonization of analytical methods was demonstrated by assessing whether average results deviated from the certified reference value of control materials within the APS derived from biological variation (BV). The APS used in EQA have evolved from state-of-the-art models to BV. Poor clinical decision-making would occur if the results accepted by some APS were applied.
Conclusions: In Spain, only 2 of the 18 measurands studied are considered to be well harmonized. Closer collaboration between laboratories and analytical system providers would be required to resolve discrepancies.
Keywords: analytical performance specifications; biological variation; external quality assurance schemes; harmonization; state of the art.
© 2020 Carmen Ricós et al., published by De Gruyter, Berlin/Boston.
Figures
References
-
- JCTLM, BIPM Database of higher order reference materials, measurement methods and services. https://www.bipm.org/jctlm/home.do
-
- Braga F, Pantegini M. Verification of in vitro medical diagnostics (IVD) metrological traceability: responsibilities and strategies. Clin Chim Acta. 2014;435:55–61. - PubMed
-
- Ceriotti F, Cobbaert C. Harmonization of external quality assessment schemes and their role – clinical chemistry and beyond. Clin Chem Lab Med. 2018;56:1587–90. - PubMed
-
- IFCC/EMD/C-AQ . Guidelines for the requirements for the competence of EQAP organizers in medical laboratories. 2002. https://www.ifcc.org/media/478095/eqap_-version-3.pdf. IFCC/EMD/C-AQ [Accessed 17 Sep 2019]
LinkOut - more resources
Full Text Sources
Miscellaneous
