A method for the synthesis of unsymmetric bisphosphoric analogs of α-amino acids
- PMID: 37362601
- PMCID: PMC10288832
- DOI: 10.1039/d3ra02981f
A method for the synthesis of unsymmetric bisphosphoric analogs of α-amino acids
Abstract
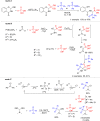
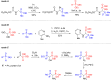
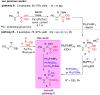
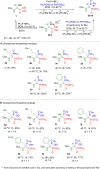
Herein, we describe the first universal strategy for the synthesis of unsymmetric phosphonyl-phosphinyl and phosphonyl-phosphinoyl analogs of N-protected 1-aminobisphosphonates. The proposed user-friendly procedure, based on a one-pot reaction of the α-ethoxy derivatives of phosphorus analogs of protein and non-protein α-amino acids with triphenylphosphonium tetrafluoroborate and an appropriate phosphorus nucleophile (diethyl phenylphosphonite or methyl diphenylphosphinite), provides good to very good yields of 53-91% under mild catalyst-free conditions (temperature: rt to 40 °C, time: 1 to 6 hours). The progress of the transformation, running through the corresponding phosphonium salt as a reactive intermediate, was monitored by 31P NMR spectroscopy, which is a convenient tool for the identification of the transient species formed here. In this paper, we present the full characteristics of the spectroscopic properties of all 13 synthesized models of structurally diverse N-protected unsymmetric bisphosphoric analogs of α-amino acids. Therefore, these results contribute to increasing the practical applicability of our recently reported synthesis protocol of symmetric models of α-aminobisphosphonates derivatives and thus justify its universality.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Aminophosphonic and aminophosphinic acids: chemistry and biological activity, ed. V. P. Kukhar', Wiley, Chichester; New York, 2000
-
- Romanenko V. D. Kukhar V. P. Arkivoc. 2012;2012:127–166.
-
- Hiraga T. Tanaka S. Yamamoto M. Nakajima T. Ozawa H. Bone. 1996;18:1–7. - PubMed
-
- Zhang S. Gangal G. Uludağ H. Chem. Soc. Rev. 2007;36:507–531. - PubMed
-
- Chmielewska E. Kafarski P. Open Pharm. Sci. J. 2016;3:56–78.
LinkOut - more resources
Full Text Sources

