Live-Cell SOFI Correlation with SMLM and AFM Imaging
- PMID: 37363082
- PMCID: PMC10288496
- DOI: 10.1021/acsbiomedchemau.2c00086
Live-Cell SOFI Correlation with SMLM and AFM Imaging
Abstract
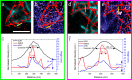
Standard optical imaging is diffraction-limited and lacks the resolving power to visualize many of the organelles and proteins found within the cell. The advent of super-resolution techniques overcame this barrier, enabling observation of subcellular structures down to tens of nanometers in size; however these techniques require or are typically applied to fixed samples. This raises the question of how well a fixed-cell image represents the system prior to fixation. Here we present the addition of live-cell Super-Resolution Optical Fluctuation Imaging (SOFI) to a previously reported correlative process using Single Molecule Localization Microscopy (SMLM) and Atomic Force Microscopy (AFM). SOFI was used with fluorescent proteins and low laser power to observe cellular ultrastructure in live COS-7 cells. SOFI-SMLM-AFM of microtubules showed minimal changes to the microtubule network in the 20 min between live-cell SOFI and fixation. Microtubule diameters were also analyzed through all microscopies; SOFI found diameters of 249 ± 68 nm and SMLM was 71 ± 33 nm. AFM height measurements found microtubules to protrude 26 ± 13 nm above the surrounding cellular material. The correlation of SMLM and AFM was extended to two-color SMLM to image both microtubules and actin. Two target SOFI was performed with various fluorescent protein combinations. rsGreen1-rsKAME, rsGreen1-Dronpa, and ffDronpaF-rsKAME fluorescent protein combinations were determined to be suitable for two target SOFI imaging. This correlative application of super-resolution live-cell and fixed-cell imaging revealed minimal artifacts created for the imaged target structures through the sample preparation procedure and emphasizes the power of correlative microscopy.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Whelan D. R.; Bell T. D. M. Correlative synchrotron fourier transform infrared spectroscopy and single molecule super resolution microscopy for the detection of composition and ultrastructure alterations in single cells. ACS Chem. Biol. 2015, 10, 2874–2883. 10.1021/acschembio.5b00754. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

