Physical pictures of rotation mechanisms of F1- and V1-ATPases: Leading roles of translational, configurational entropy of water
- PMID: 37363397
- PMCID: PMC10288849
- DOI: 10.3389/fmolb.2023.1159603
Physical pictures of rotation mechanisms of F1- and V1-ATPases: Leading roles of translational, configurational entropy of water
Abstract
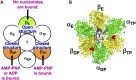
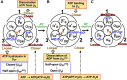
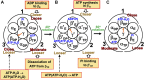
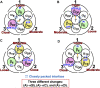
We aim to develop a theory based on a concept other than the chemo-mechanical coupling (transduction of chemical free energy of ATP to mechanical work) for an ATP-driven protein complex. Experimental results conflicting with the chemo-mechanical coupling have recently emerged. We claim that the system comprises not only the protein complex but also the aqueous solution in which the protein complex is immersed and the system performs essentially no mechanical work. We perform statistical-mechanical analyses on V1-ATPase (the A3B3DF complex) for which crystal structures in more different states are experimentally known than for F1-ATPase (the α3β3γ complex). Molecular and atomistic models are employed for water and the structure of V1-ATPase, respectively. The entropy originating from the translational displacement of water molecules in the system is treated as a pivotal factor. We find that the packing structure of the catalytic dwell state of V1-ATPase is constructed by the interplay of ATP bindings to two of the A subunits and incorporation of the DF subunit. The packing structure represents the nonuniformity with respect to the closeness of packing of the atoms in constituent proteins and protein interfaces. The physical picture of rotation mechanism of F1-ATPase recently constructed by Kinoshita is examined, and common points and differences between F1- and V1-ATPases are revealed. An ATP hydrolysis cycle comprises binding of ATP to the protein complex, hydrolysis of ATP into ADP and Pi in it, and dissociation of ADP and Pi from it. During each cycle, the chemical compounds bound to the three A or β subunits and the packing structure of the A3B3 or α3β3 complex are sequentially changed, which induces the unidirectional rotation of the central shaft for retaining the packing structure of the A3B3DF or α3β3γ complex stabilized for almost maximizing the water entropy. The torque driving the rotation is generated by water with no input of chemical free energy. The presence of ATP is indispensable as a trigger of the torque generation. The ATP hydrolysis or synthesis reaction is tightly coupled to the rotation of the central shaft in the normal or inverse direction through the water-entropy effect.
Keywords: ATP hydrolysis; ATP synthesis; ATP-driven protein; linear molecular motor; protein structure; rotary molecular motor; statistical mechanics; water entropy.
Copyright © 2023 Yasuda, Hayashi, Murata and Kinoshita.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

