The impact of engineered nickel oxide nanoparticles on ascorbate glutathione cycle in Allium cepa L
- PMID: 37363417
- PMCID: PMC10284763
- DOI: 10.1007/s12298-023-01314-8
The impact of engineered nickel oxide nanoparticles on ascorbate glutathione cycle in Allium cepa L
Abstract
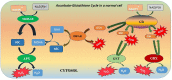
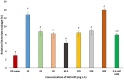
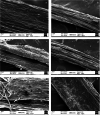
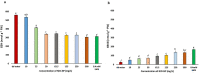
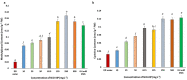
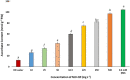
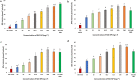
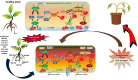
Engineered nickel oxide nanoparticle (NiO-NP) can inflict significant damages on exposed plants, even though very little is known about the modus operandi. The present study investigated effects of NiO-NP on the crucial stress alleviation mechanism Ascorbate-Glutathione Cycle (Asa-GSH cycle) in the model plant Allium cepa. Cellular contents of reduced glutathione (GSH) and oxidised glutathione (GSSG), was disturbed upon NiO-NP exposure. The ratio of GSH to GSSG changed from 20:1 in NC to 4:1 in roots exposed to 125 mg L-1 NiO-NP. Even the lowest treatments of NiO-NP (10 mg L-1) increased ascorbic acid (2.9-folds) and cysteine contents (1.6-folds). Enzymes like glutathione reductase, ascorbate peroxidase, glutathione peroxidase and glutathione-S-transferase also showed altered activities in the affected tissues. Further, intracellular methylglyoxal, a harbinger of ROS (Reactive oxygen species), increased significantly (~ 26 to 65-fold) across different concentrations NiO-NP. Intracellular H2O2 (hydrogen peroxide) and ROS levels increased with NiO-NP doses, as did electrolytic leakage from damaged cells. The present work indicated that multiple pathways were compromised in NiO-NP affected plants and this information can bolster our general understanding of the actual mechanism of its toxicity on living cells, and help formulate strategies to thwart ecological pollution.
Supplementary information: The online version contains supplementary material available at 10.1007/s12298-023-01314-8.
Keywords: Antioxidants; Engineered nanoparticle hazard; Environmental pollutant; GSH:GSSG ratio; Methylglyoxal; ROS; Reduced glutathione.
© Prof. H.S. Srivastava Foundation for Science and Society 2023. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Conflict of interestThe authors would like to declare that there is no conflict of competing interest, and no commercial or financial aid which can be construed as a conflicting interest, has been involved in the study.
Figures

Similar articles
-
Effect of Engineered Nickel Oxide Nanoparticle on Reactive Oxygen Species-Nitric Oxide Interplay in the Roots of Allium cepa L.Front Plant Sci. 2021 Feb 9;12:586509. doi: 10.3389/fpls.2021.586509. eCollection 2021. Front Plant Sci. 2021. PMID: 33633755 Free PMC article.
-
Engineered nickel oxide nanoparticles affect genome stability in Allium cepa (L.).Plant Physiol Biochem. 2017 Dec;121:206-215. doi: 10.1016/j.plaphy.2017.11.003. Epub 2017 Nov 8. Plant Physiol Biochem. 2017. PMID: 29136573
-
Engineered Nickel Oxide Nanoparticle Causes Substantial Physicochemical Perturbation in Plants.Front Chem. 2017 Nov 8;5:92. doi: 10.3389/fchem.2017.00092. eCollection 2017. Front Chem. 2017. PMID: 29167790 Free PMC article.
-
Responses of individual and combined polystyrene and polymethyl methacrylate nanoplastics on hormonal content, fluorescence/photochemistry of chlorophylls and ROS scavenging capacity in Lemna minor under arsenic-induced oxidative stress.Free Radic Biol Med. 2023 Feb 20;196:93-107. doi: 10.1016/j.freeradbiomed.2023.01.015. Epub 2023 Jan 16. Free Radic Biol Med. 2023. PMID: 36657731 Review.
-
The pivotal function of dehydroascorbate reductase in glutathione homeostasis in plants.J Exp Bot. 2020 Jun 22;71(12):3405-3416. doi: 10.1093/jxb/eraa107. J Exp Bot. 2020. PMID: 32107543 Review.
References
-
- Ahmad P, Ozturk M, Gucel S. Oxidative damage and antioxidants induced by heavy metal stress in two cultivars of mustard (Brassica juncea L.) plants. Fresenius Environ Bull. 2012;21(10):2953–2961. doi: 10.1078/0176-1617-00833. - DOI
-
- Ando K, Honma M, Chiba S, Tahara S, Mizutani J. Glutathione transferase from Mucor javanicus. Agric Biol Chem. 1988;52(1):135–139. doi: 10.1271/bbb1961.52.135. - DOI
-
- Anjum NA, Singh N, Singh MK, Shah ZA, Duarte AC, Pereira E, Ahmad I. Single-bilayer graphene oxide sheet tolerance and glutathione redox system significance assessment in faba bean (Vicia faba L.) J Nanoparticle Res. 2013;15(7):1770. doi: 10.1007/s11051-013-1770-7. - DOI
-
- Asada K. Ascorbate peroxidase–a hydrogen peroxide-scavenging enzyme in plants. Physiol Plant. 1992;85(2):235–241. doi: 10.1111/j.1399-3054.1992.tb04728.x. - DOI
-
- Baccouch M, Choura S, El-Borgi S, Nayfeh AH. Selection of physical and geometrical properties for the confinement of vibrations in nonhomogeneous beams. J Aerosp Eng. 2006;19(3):158–168. doi: 10.1061/(ASCE)0893-1321(2006)19:3(158). - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
