Critical Review on Bromate Formation during Ozonation and Control Options for Its Minimization
- PMID: 37363871
- PMCID: PMC10690720
- DOI: 10.1021/acs.est.3c00538
Critical Review on Bromate Formation during Ozonation and Control Options for Its Minimization
Abstract
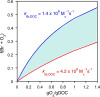
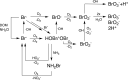
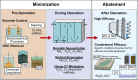
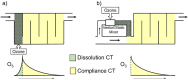
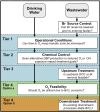
Ozone is a commonly applied disinfectant and oxidant in drinking water and has more recently been implemented for enhanced municipal wastewater treatment for potable reuse and ecosystem protection. One drawback is the potential formation of bromate, a possible human carcinogen with a strict drinking water standard of 10 μg/L. The formation of bromate from bromide during ozonation is complex and involves reactions with both ozone and secondary oxidants formed from ozone decomposition, i.e., hydroxyl radical. The underlying mechanism has been elucidated over the past several decades, and the extent of many parallel reactions occurring with either ozone or hydroxyl radicals depends strongly on the concentration, type of dissolved organic matter (DOM), and carbonate. On the basis of mechanistic considerations, several approaches minimizing bromate formation during ozonation can be applied. Removal of bromate after ozonation is less feasible. We recommend that bromate control strategies be prioritized in the following order: (1) control bromide discharge at the source and ensure optimal ozone mass-transfer design to minimize bromate formation, (2) minimize bromate formation during ozonation by chemical control strategies, such as ammonium with or without chlorine addition or hydrogen peroxide addition, which interfere with specific bromate formation steps and/or mask bromide, (3) implement a pretreatment strategy to reduce bromide and/or DOM prior to ozonation, and (4) assess the suitability of ozonation altogether or utilize a downstream treatment process that may already be in place, such as reverse osmosis, for post-ozone bromate abatement. A one-size-fits-all approach to bromate control does not exist, and treatment objectives, such as disinfection and micropollutant abatement, must also be considered.
Keywords: bromate; dissolved organic matter; human carcinogen; ozonation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- von Sonntag C.; von Gunten U.. Chemistry of Ozone in Water and Wastewater Treatment: From Basic Principles to Applications; IWA Publishing, 2012. 10.2166/9781780400839 - DOI
-
- Oneby M. A.; Bromley C. O.; Borchardt J. H.; Harrison D. S. Ozone treatment of secondary effluent at U.S. municipal wastewater treatment plants. Ozone Sci. Eng. 2010, 32, 43–55. 10.1080/01919510903482780. - DOI
-
- Stage 1 Disinfectants and Disinfection Byproduct Rule (Stage 1 DBPR) 63 FR 69390. U.S. Environmental Protection Agency, 1998; 63, No. (241), .
-
- Guidelines for Drinking Water Quality, 4th ed. (incorporating the first and second addenda); World Health Organization: Geneva, 2022. - PubMed
-
- Kurokawa Y.; Aoki S.; Matsushima Y.; Takamura N.; Imazawa T.; Hayashi Y. Dose-response studies on the carcinogenicity of potassium bromate in F344 rats after long-term oral administration. J. Natl. Cancer Inst. 1986, 77, 977–982. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

