Safety and Efficacy of Tucatinib, Letrozole, and Palbociclib in Patients with Previously Treated HR+/HER2+ Breast Cancer
- PMID: 37363965
- PMCID: PMC10722138
- DOI: 10.1158/1078-0432.CCR-23-0117
Safety and Efficacy of Tucatinib, Letrozole, and Palbociclib in Patients with Previously Treated HR+/HER2+ Breast Cancer
Abstract
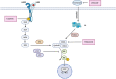
Purpose: To overcome resistance to antihormonal and HER2-targeted agents mediated by cyclin D1-CDK4/6 complex, we proposed an oral combination of the HER2 inhibitor tucatinib, aromatase inhibitor letrozole, and CDK4/6 inhibitor palbociclib (TLP combination) for treatment of HR+/HER2+ metastatic breast cancer (MBC).
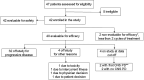
Patients and methods: Phase Ib/II TLP trial (NCT03054363) enrolled patients with HR+/HER2+ MBC treated with ≥2 HER2-targeted agents. The phase Ib primary endpoint was safety of the regimen evaluated by NCI CTCAE version 4.3. The phase II primary endpoint was efficacy by median progression-free survival (mPFS).
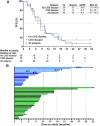
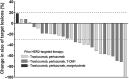
Results: Forty-two women ages 22 to 81 years were enrolled. Patients received a median of two lines of therapy in the metastatic setting, 71.4% had visceral disease, 35.7% had CNS disease. The most common treatment-emergent adverse events (AE) of grade ≥3 were neutropenia (64.3%), leukopenia (23.8%), diarrhea (19.0%), and fatigue (14.3%). Tucatinib increased AUC10-19 hours of palbociclib 1.7-fold, requiring palbociclib dose reduction from 125 to 75 mg daily. In 40 response-evaluable patients, mPFS was 8.4 months, with similar mPFS in non-CNS and CNS cohorts (10.0 months vs. 8.2 months; P = 0.9). Overall response rate was 44.5%, median duration of response was 13.9 months, and clinical benefit rate was 70.4%; 60% of patients were on treatment for ≥6 months, 25% for ≥1 year, and 10% for ≥2 years. In the CNS cohort, 26.6% of patients remained on study for ≥1 year.
Conclusions: TLP combination was safe and tolerable. AEs were expected and manageable with supportive therapy and dose reductions. TLP showed excellent efficacy for an all-oral chemotherapy-free regimen warranting further testing. See related commentary by Huppert and Rugo, p. 4993.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures
Comment in
-
Can We De-escalate Therapy for HER2-Positive Metastatic Breast Cancer?Clin Cancer Res. 2023 Dec 15;29(24):4993-4995. doi: 10.1158/1078-0432.CCR-23-1909. Clin Cancer Res. 2023. PMID: 37782311
References
-
- American Cancer Society. Breast cancer facts & figures. Atlanta, GA: American Cancer Society. p. v.
-
- Montemurro F, Di Cosimo S, Arpino G. Human epidermal growth factor receptor 2 (HER2)-positive and hormone receptor-positive breast cancer: new insights into molecular interactions and clinical implications. Ann Oncol 2013;24:2715–24. - PubMed
-
- Cortes J, Baselga J. How to treat hormone receptor-positive, human epidermal growth factor receptor 2-amplified breast cancer. J Clin Oncol 2009;27:5492–4. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

