Safety, Efficacy, and Biomarker Analyses of Dostarlimab in Patients with Endometrial Cancer: Interim Results of the Phase I GARNET Study
- PMID: 37363992
- PMCID: PMC10643997
- DOI: 10.1158/1078-0432.CCR-22-3915
Safety, Efficacy, and Biomarker Analyses of Dostarlimab in Patients with Endometrial Cancer: Interim Results of the Phase I GARNET Study
Abstract
Purpose: This interim report of the GARNET phase I trial presents efficacy and safety of dostarlimab in patients with advanced or recurrent endometrial cancer (EC), with an analysis of tumor biomarkers as prognostic indicators.
Patients and methods: A total of 153 patients with mismatch repair deficient (dMMR)/microsatellite instability-high (MSI-H) and 161 patients with mismatch repair proficient (MMRp)/microsatellite stable (MSS) EC were enrolled and dosed. Patients received 500 mg dostarlimab every 3 weeks for four cycles, then 1,000 mg every 6 weeks until progression. Primary endpoints were objective response rate (ORR) and duration of response (DOR).
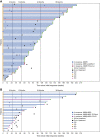
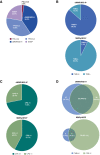
Results: A total of 143 patients with dMMR/MSI-H EC and 156 patients with MMRp/MSS EC were evaluated for efficacy. ORR was 45.5% (n = 65) and 15.4% (n = 24) for dMMR/MSI-H EC and MMRp/MSS EC, respectively. Median DOR for dMMR/MSI-H EC was not met (median follow-up, 27.6 months); median DOR for MMRp/MSS EC was 19.4 months. The ORRs by combined positive score (CPS) ≥1 status were 54.9% and 21.7% for dMMR/MSI-H EC and MMRp/MSS EC, respectively. ORRs by high tumor mutational burden (≥10 mutations/Mb) were 47.8% (43/90) and 45.5% (5/11) for dMMR/MSI-H EC and MMRp/MSS EC, respectively. ORR in TP53mut or POLεmut molecular subgroups was 18.1% (17/94) and 40.0% (2/5), respectively. The safety profile of dostarlimab was consistent with previous reports.
Conclusions: Dostarlimab demonstrated durable antitumor activity and safety in patients with dMMR/MSI-H EC. Biomarkers associated with EC may identify patients likely to respond to dostarlimab. See related commentary by Jangra and Dhani, p. 4521.
Trial registration: ClinicalTrials.gov NCT02715284.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures

Comment in
-
Rerouting the GPS Directing Immunotherapy in Endometrial Cancer.Clin Cancer Res. 2023 Nov 14;29(22):4521-4523. doi: 10.1158/1078-0432.CCR-23-1953. Clin Cancer Res. 2023. PMID: 37698948
References
-
- Gu B, Shang X, Yan M, Li X, Wang W, Wang Qet al. . Variations in incidence and mortality rates of endometrial cancer at the global, regional, and national levels, 1990–2019. Gynecol Oncol 2021;161:573–80. - PubMed
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7–33. - PubMed
-
- Arend RC, Jones BA, Martinez A, Goodfellow P. Endometrial cancer: molecular markers and management of advanced stage disease. Gynecol Oncol 2018;150:569–80. - PubMed
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

