Ultrafast Bioorthogonal Spin-Labeling and Distance Measurements in Mammalian Cells Using Small, Genetically Encoded Tetrazine Amino Acids
- PMID: 37364003
- PMCID: PMC10440187
- DOI: 10.1021/jacs.3c00967
Ultrafast Bioorthogonal Spin-Labeling and Distance Measurements in Mammalian Cells Using Small, Genetically Encoded Tetrazine Amino Acids
Abstract
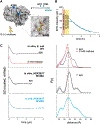
Site-directed spin-labeling (SDSL)─in combination with double electron-electron resonance (DEER) spectroscopy─has emerged as a powerful technique for determining both the structural states and the conformational equilibria of biomacromolecules. DEER combined with in situ SDSL in live cells is challenging since current bioorthogonal labeling approaches are too slow to allow for complete labeling with low concentrations of spin label prior to loss of signal from cellular reduction. Here, we overcome this limitation by genetically encoding a novel family of small, tetrazine-bearing noncanonical amino acids (Tet-v4.0) at multiple sites in proteins expressed in Escherichia coli and in human HEK293T cells. We achieved specific and quantitative spin-labeling of Tet-v4.0-containing proteins by developing a series of strained trans-cyclooctene (sTCO)-functionalized nitroxides─including a gem-diethyl-substituted nitroxide with enhanced stability in cells─with rate constants that can exceed 106 M-1 s-1. The remarkable speed of the Tet-v4.0/sTCO reaction allowed efficient spin-labeling of proteins in live cells within minutes, requiring only sub-micromolar concentrations of sTCO-nitroxide. DEER recorded from intact cells revealed distance distributions in good agreement with those measured from proteins purified and labeled in vitro. Furthermore, DEER was able to resolve the maltose-dependent conformational change of Tet-v4.0-incorporated and spin-labeled MBP in vitro and support assignment of the conformational state of an MBP mutant within HEK293T cells. We anticipate the exceptional reaction rates of this system, combined with the relatively short and rigid side chains of the resulting spin labels, will enable structure/function studies of proteins directly in cells, without any requirements for protein purification.
Figures

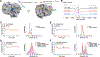

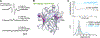
Update of
-
Ultra-Fast Bioorthogonal Spin-Labeling and Distance Measurements in Mammalian Cells Using Small, Genetically Encoded Tetrazine Amino Acids.bioRxiv [Preprint]. 2023 Jan 31:2023.01.26.525763. doi: 10.1101/2023.01.26.525763. bioRxiv. 2023. Update in: J Am Chem Soc. 2023 Jul 12;145(27):14608-14620. doi: 10.1021/jacs.3c00967. PMID: 36747808 Free PMC article. Updated. Preprint.
References
-
- Theillet F-X, Binolfi A, Frembgen-Kesner T, Hingorani K, Sarkar M, Kyne C, Li C, Crowley PB, Gierasch L, Pielak GJ, et al. (2014). Physicochemical Properties of Cells and Their Effects on Intrinsically Disordered Proteins (IDPs). Chemical Reviews 114, 6661–6714. 10.1021/cr400695p. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

