Role of the ST6GAL1 sialyltransferase in regulating ovarian cancer cell metabolism
- PMID: 37364046
- PMCID: PMC10560082
- DOI: 10.1093/glycob/cwad051
Role of the ST6GAL1 sialyltransferase in regulating ovarian cancer cell metabolism
Abstract
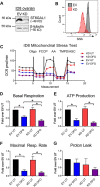
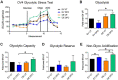
The ST6GAL1 sialyltransferase, which adds α2-6-linked sialic acids to N-glycosylated proteins, is upregulated in many malignancies including ovarian cancer. Through its activity in sialylating select surface receptors, ST6GAL1 modulates intracellular signaling to regulate tumor cell phenotype. ST6GAL1 has previously been shown to act as a survival factor that protects cancer cells from cytotoxic stressors such as hypoxia. In the present study, we investigated a role for ST6GAL1 in tumor cell metabolism. ST6GAL1 was overexpressed (OE) in OV4 ovarian cancer cells, which have low endogenous ST6GAL1, or knocked-down (KD) in ID8 ovarian cancer cells, which have high endogenous ST6GAL1. OV4 and ID8 cells with modulated ST6GAL1 expression were grown under normoxic or hypoxic conditions, and metabolism was assessed using Seahorse technology. Results showed that cells with high ST6GAL1 expression maintained a higher rate of oxidative metabolism than control cells following treatment with the hypoxia mimetic, desferrioxamine (DFO). This enrichment was not due to an increase in mitochondrial number. Glycolytic metabolism was also increased in OV4 and ID8 cells with high ST6GAL1 expression, and these cells displayed greater activity of the glycolytic enzymes, hexokinase and phosphofructokinase. Metabolism maps were generated from the combined Seahorse data, which suggested that ST6GAL1 functions to enhance the overall metabolism of tumor cells. Finally, we determined that OV4 and ID8 cells with high ST6GAL1 expression were more invasive under conditions of hypoxia. Collectively, these results highlight the importance of sialylation in regulating the metabolic phenotype of ovarian cancer cells.
Keywords: ST6GAL1; cancer stem cells; hypoxia; metabolism; sialic acid.
© The Author(s) 2023. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures






References
-
- Aasheim HC, Aas-Eng DA, Deggerdal A, Blomhoff HK, Funderud S, Smeland EB. Cell-specific expression of human beta-galactoside alpha 2,6-sialyltransferase transcripts differing in the 5′ untranslated region. Eur J Biochem. 1993:213(1):467–475. - PubMed
-
- Ahmed N, Escalona R, Leung D, Chan E, Kannourakis G. Tumour microenvironment and metabolic plasticity in cancer and cancer stem cells: perspectives on metabolic and immune regulatory signatures in chemoresistant ovarian cancer stem cells. Semin Cancer Biol. 2018:53:265–281. - PubMed
-
- Bellis SL, Reis CA, Varki A, Kannagi R, Stanley P. Glycosylation changes in cancer. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Kinoshita T, Mohnen D, Packer NH, Prestegard JH, et al., editors. Essentials of glycobiology, Chapter 47. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2022. pp. 631–644
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

