Nanoscale β-TCP-Laden GelMA/PCL Composite Membrane for Guided Bone Regeneration
- PMID: 37364054
- PMCID: PMC10982892
- DOI: 10.1021/acsami.3c03059
Nanoscale β-TCP-Laden GelMA/PCL Composite Membrane for Guided Bone Regeneration
Abstract
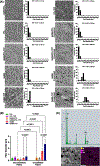
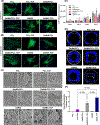
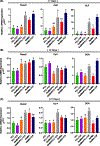
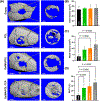
Major advances in the field of periodontal tissue engineering have favored the fabrication of biodegradable membranes with tunable physical and biological properties for guided bone regeneration (GBR). Herein, we engineered innovative nanoscale beta-tricalcium phosphate (β-TCP)-laden gelatin methacryloyl/polycaprolactone (GelMA/PCL-TCP) photocrosslinkable composite fibrous membranes via electrospinning. Chemo-morphological findings showed that the composite microfibers had a uniform porous network and β-TCP particles successfully integrated within the fibers. Compared with pure PCL and GelMA/PCL, GelMA/PCL-TCP membranes led to increased cell attachment, proliferation, mineralization, and osteogenic gene expression in alveolar bone-derived mesenchymal stem cells (aBMSCs). Moreover, our GelMA/PCL-TCP membrane was able to promote robust bone regeneration in rat calvarial critical-size defects, showing remarkable osteogenesis compared to PCL and GelMA/PCL groups. Altogether, the GelMA/PCL-TCP composite fibrous membrane promoted osteogenic differentiation of aBMSCs in vitro and pronounced bone formation in vivo. Our data confirmed that the electrospun GelMA/PCL-TCP composite has a strong potential as a promising membrane for guided bone regeneration.
Keywords: bone; electrospinning; extracellular matrix; gelatin; regeneration; tissue engineering.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Evaluation of 3D printed PCL/PLGA/β-TCP versus collagen membranes for guided bone regeneration in a beagle implant model.Biomed Mater. 2016 Oct 7;11(5):055013. doi: 10.1088/1748-6041/11/5/055013. Biomed Mater. 2016. PMID: 27716630
-
Enhanced osteogenesis and angiogenesis by PCL/chitosan/Sr-doped calcium phosphate electrospun nanocomposite membrane for guided bone regeneration.J Biomater Sci Polym Ed. 2019 Nov;30(16):1505-1522. doi: 10.1080/09205063.2019.1646628. Epub 2019 Aug 13. J Biomater Sci Polym Ed. 2019. PMID: 31322979
-
Efficacy of rhBMP-2 Loaded PCL/β-TCP/bdECM Scaffold Fabricated by 3D Printing Technology on Bone Regeneration.Biomed Res Int. 2018 Feb 27;2018:2876135. doi: 10.1155/2018/2876135. eCollection 2018. Biomed Res Int. 2018. PMID: 29682530 Free PMC article.
-
Periosteum and development of the tissue-engineered periosteum for guided bone regeneration.J Orthop Translat. 2022 Feb 16;33:41-54. doi: 10.1016/j.jot.2022.01.002. eCollection 2022 Mar. J Orthop Translat. 2022. PMID: 35228996 Free PMC article. Review.
-
Leveraging the Recent Advancements in GelMA Scaffolds for Bone Tissue Engineering: An Assessment of Challenges and Opportunities.Biomacromolecules. 2024 Apr 8;25(4):2075-2113. doi: 10.1021/acs.biomac.3c00279. Epub 2023 Jul 5. Biomacromolecules. 2024. PMID: 37406611 Review.
Cited by
-
Addressing the challenges of infectious bone defects: a review of recent advances in bifunctional biomaterials.J Nanobiotechnology. 2025 Mar 29;23(1):257. doi: 10.1186/s12951-025-03295-0. J Nanobiotechnology. 2025. PMID: 40158189 Free PMC article. Review.
-
E7-Conjugated Bio-Inspired Microspheres as a Biological Barrier for Guided Tissue Regeneration.ACS Appl Mater Interfaces. 2023 Dec 20;15(50):58136-58150. doi: 10.1021/acsami.3c12213. Epub 2023 Dec 8. ACS Appl Mater Interfaces. 2023. PMID: 38063848 Free PMC article.
-
Macrophage membrane functionalized composite microspheres promote bone regeneration in periodontitis via manipulating inflammation reversing-osteogenesis coupling.Mater Today Bio. 2025 Apr 22;32:101789. doi: 10.1016/j.mtbio.2025.101789. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40331151 Free PMC article.
-
A Bioactive Gelatin-Methacrylate Incorporating Magnesium Phosphate Cement for Bone Regeneration.Biomedicines. 2024 Jan 19;12(1):228. doi: 10.3390/biomedicines12010228. Biomedicines. 2024. PMID: 38275399 Free PMC article.
-
Personalized bioceramic grafts for craniomaxillofacial bone regeneration.Int J Oral Sci. 2024 Oct 31;16(1):62. doi: 10.1038/s41368-024-00327-7. Int J Oral Sci. 2024. PMID: 39482290 Free PMC article. Review.
References
-
- Cobb CM; Sottosanti JS A Re-Evaluation of Scaling and Root Planing. J. Periodontol. 2021, 92, 1370–1378. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

