Ensifentrine, a Novel Phosphodiesterase 3 and 4 Inhibitor for the Treatment of Chronic Obstructive Pulmonary Disease: Randomized, Double-Blind, Placebo-controlled, Multicenter Phase III Trials (the ENHANCE Trials)
- PMID: 37364283
- PMCID: PMC10449067
- DOI: 10.1164/rccm.202306-0944OC
Ensifentrine, a Novel Phosphodiesterase 3 and 4 Inhibitor for the Treatment of Chronic Obstructive Pulmonary Disease: Randomized, Double-Blind, Placebo-controlled, Multicenter Phase III Trials (the ENHANCE Trials)
Abstract
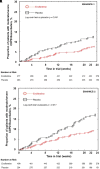
Rationale: Ensifentrine is a novel, selective, dual phosphodiesterase (PDE)3 and PDE4 inhibitor with bronchodilator and antiinflammatory effects. Replicate phase III trials of nebulized ensifentrine were conducted (ENHANCE-1 and ENHANCE-2) to assess these effects in patients with chronic obstructive pulmonary disease (COPD). Objectives: To evaluate the efficacy of ensifentrine compared with placebo for lung function, symptoms, quality of life, and exacerbations in patients with COPD. Methods: These phase III, multicenter, randomized, double-blind, parallel-group, placebo-controlled trials were conducted between September 2020 and December 2022 at 250 research centers and pulmonology practices in 17 countries. Patients aged 40-80 years with moderate to severe symptomatic COPD were enrolled. Measurements and Main Results: Totals of 760 (ENHANCE-1) and 789 (ENHANCE-2) patients were randomized and treated, with 69% and 55% receiving concomitant long-acting muscarinic antagonists or long-acting β2-agonists, respectively. Post-bronchodilator FEV1 percentage predicted values were 52% and 51% of predicted normal. Ensifentrine treatment significantly improved average FEV1 area under the curve at 0-12 hours versus placebo (ENHANCE-1, 87 ml [95% confidence interval, 55, 119]; ENHANCE-2, 94 ml [65, 124]; both P < 0.001). Ensifentrine treatment significantly improved symptoms (Evaluating Respiratory Symptoms) and quality of life (St. George's Respiratory Questionnaire) versus placebo at Week 24 in ENHANCE-1 but not in ENHANCE-2. Ensifentrine treatment reduced the rate of moderate or severe exacerbations versus placebo over 24 weeks (ENHANCE-1, rate ratio, 0.64 [0.40, 1.00]; P = 0.050; ENHANCE-2, rate ratio, 0.57 [0.38, 0.87]; P = 0.009) and increased time to first exacerbation (ENHANCE-1, hazard ratio, 0.62 [0.39, 0.97]; P = 0.038; ENHANCE-2, hazard ratio, 0.58 [0.38, 0.87]; P = 0.009). Adverse event rates were similar to those for placebo. Conclusions: Ensifentrine significantly improved lung function in both trials, with results supporting exacerbation rate and risk reduction in a broad COPD population and in addition to other classes of maintenance therapies. Clinical trial registered with www.
Clinicaltrials: gov and EudraCT (ENHANCE-1, www.
Clinicaltrials: gov identifier NCT04535986, EudraCT identifier 2020-002086-34; ENHANCE-2, www.
Clinicaltrials: gov identifier NCT04542057, EudraCT identifier 2020-002069-32).
Keywords: COPD; dual PDE3 and PDE4 inhibitor; ensifentrine; nebulized therapy.
Figures

Comment in
-
A New Treatment for Chronic Obstructive Pulmonary Disease: Ensifentrine Moves Closer.Am J Respir Crit Care Med. 2023 Aug 15;208(4):344-346. doi: 10.1164/rccm.202307-1164ED. Am J Respir Crit Care Med. 2023. PMID: 37433204 Free PMC article. No abstract available.
-
Pharmacological Interpretation of the Efficacy of Ensifentrine in Chronic Obstructive Pulmonary Disease: Insights from ENHANCE Trials.Am J Respir Crit Care Med. 2024 Jan 15;209(2):224-225. doi: 10.1164/rccm.202308-1355LE. Am J Respir Crit Care Med. 2024. PMID: 37939379 Free PMC article. No abstract available.
-
Still Thirsty in COPD!Am J Respir Crit Care Med. 2024 Jan 15;209(2):225-226. doi: 10.1164/rccm.202309-1605LE. Am J Respir Crit Care Med. 2024. PMID: 37939380 Free PMC article. No abstract available.
-
Inhaled Dual PDE3/4 Inhibitor Ensifentrine for Chronic Obstructive Pulmonary Disease: A Potential Therapeutic Perspective.Am J Respir Crit Care Med. 2024 Jan 15;209(2):223-224. doi: 10.1164/rccm.202307-1143LE. Am J Respir Crit Care Med. 2024. PMID: 37939381 Free PMC article. No abstract available.
References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2023. https://goldcopd.org/2023-gold-report-2/
-
- Phreesia Life Sciences. Patients in focus: COPD treatment and perceptions. Wilmington, DE: Phreesia Life Sciences; 2023.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

