Pembrolizumab Every 6 Weeks Versus Every 3 Weeks in Advanced Non-Small Cell Lung Cancer
- PMID: 37364568
- PMCID: PMC10628563
- DOI: 10.1093/oncolo/oyad182
Pembrolizumab Every 6 Weeks Versus Every 3 Weeks in Advanced Non-Small Cell Lung Cancer
Abstract
Background: The survival benefits and adverse effects of pembrolizumab 2 mg/kg intravenously (IV) every 3 weeks (Q3W) in advanced non-small lung cancer (NSCLC) are well documented in the literature. Based on pharmacokinetic models, a pembrolizumab 4 mg/kg IV every 6 weeks (Q6W) dosing regimen is also approved in some countries. To date, there is no direct comparison in the literature between these 2 regimens in advanced NSCLC.
Methods: This retrospective study included 80 patients with advanced NSCLC who received pembrolizumab monotherapy 4 mg/kg Q6W between March 1, 2020 and December 31, 2021 and 80 patients with advanced NSCLC who received pembrolizumab monotherapy 2 mg/kg Q3W between January 1, 2017 and January 15, 2019 at Institut universitaire de cardiologie et de pneumologie de Québec (IUCPQ). The primary outcomes of this study were to compare overall survival (OS), progression-free survival (PFS) as well as the occurrence and severity of immune-mediated adverse events (AEs) in patients with advanced NSCLC who received pembrolizumab Q6W vs Q3W. Data cutoff date was December 15, 2022.
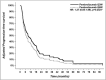
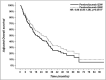
Results: Median follow-up was 14.5 ± 8.6 months in the Q6W group and 18.3 ± 19.6 months in the Q3W group. Median PFS was 6.9 months (CI 95% 5.0-10.7) in the Q6W group vs 8.9 months (CI 95% 5.6-14.1) in the Q3W group (adjusted HR 1.27 (CI 95% 0.85-1.89), P = .25). Median OS was not reached in the Q6W group vs 20.5 months (CI 95% 13.7-29.8) in the Q3W group (adjusted HR 0.80 (CI 95% 0.50-1.29), P = .36). Immune-mediated AEs of grade ≥ 3 occurred in 18% of patients in the Q6W group and in 19% of those in the Q3W group.
Conclusions: In this unicentric retrospective study, the pembrolizumab Q6W dosing regimen was comparable to the Q3W in terms of OS, PFS, and toxicity.
Keywords: advanced non-small cell lung cancer; pembrolizumab 2 mg/kg every 3 weeks; pembrolizumab 4 mg/kg every 6 weeks.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
Catherine Labbé has received honoraria from Amgen, AstraZeneca, Brystol-Myers Squibb, Merck, Pfizer, and Roche. She has also received payment for expert testimony from Jazz Pharmaceuticals, Pfizer, and Roche. She has also been on advisory boards for Amgen, Astra Zeneca, Brystol-Myers Squibb, Jazz Pharmaceuticals, LEO Pharma, Lilly, Merck, Novartis, Pfizer, Roche, and Sanofi Genzyme. The other authors indicated no financial relationships.
Figures

References
-
- Merck Canada Inc. Product monograph: Keytruda (pembrolizumab). 2020150 pp.
-
- European Medicines Agency. Annex 1: summary of product characteristics. 2019:166 pp.
-
- Medsafe. New Zealand Medecines and Medical Devices Safety Authority. New Zealand Datasheet: Keytruda (pembrolizumab). 2020:63 pp.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

