Nicotinamide Adenine Dinucleotide in Aging Biology: Potential Applications and Many Unknowns
- PMID: 37364580
- PMCID: PMC12102727
- DOI: 10.1210/endrev/bnad019
Nicotinamide Adenine Dinucleotide in Aging Biology: Potential Applications and Many Unknowns
Abstract
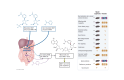
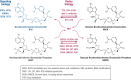
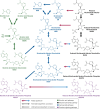
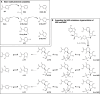
Recent research has unveiled an expansive role of NAD+ in cellular energy generation, redox reactions, and as a substrate or cosubstrate in signaling pathways that regulate health span and aging. This review provides a critical appraisal of the clinical pharmacology and the preclinical and clinical evidence for therapeutic effects of NAD+ precursors for age-related conditions, with a particular focus on cardiometabolic disorders, and discusses gaps in current knowledge. NAD+ levels decrease throughout life; age-related decline in NAD+ bioavailability has been postulated to be a contributor to many age-related diseases. Raising NAD+ levels in model organisms by administration of NAD+ precursors improves glucose and lipid metabolism; attenuates diet-induced weight gain, diabetes, diabetic kidney disease, and hepatic steatosis; reduces endothelial dysfunction; protects heart from ischemic injury; improves left ventricular function in models of heart failure; attenuates cerebrovascular and neurodegenerative disorders; and increases health span. Early human studies show that NAD+ levels can be raised safely in blood and some tissues by oral NAD+ precursors and suggest benefit in preventing nonmelanotic skin cancer, modestly reducing blood pressure and improving lipid profile in older adults with obesity or overweight; preventing kidney injury in at-risk patients; and suppressing inflammation in Parkinson disease and SARS-CoV-2 infection. Clinical pharmacology, metabolism, and therapeutic mechanisms of NAD+ precursors remain incompletely understood. We suggest that these early findings provide the rationale for adequately powered randomized trials to evaluate the efficacy of NAD+ augmentation as a therapeutic strategy to prevent and treat metabolic disorders and age-related conditions.
Keywords: NAD; NAD boosters; NAD metabolism; NAD precursors; aging; clinical applications of NAD boosters; geroscience; mechanisms of aging; pharmacologic approaches for augmenting NAD; pharmacology of NAD boosters.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

