Sperm-fluid-cell interplays in the bovine oviduct: glycosaminoglycans modulate sperm binding to the isthmic reservoir
- PMID: 37365288
- PMCID: PMC10293210
- DOI: 10.1038/s41598-023-37469-3
Sperm-fluid-cell interplays in the bovine oviduct: glycosaminoglycans modulate sperm binding to the isthmic reservoir
Abstract
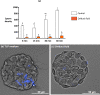
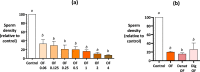
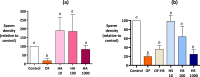
When entering the oviduct for fertilisation, spermatozoa come into contact with the oviduct fluid (OF) and can bind to luminal epithelial cells in the isthmus to form a sperm reservoir. The objective of this study was to examine how the OF modulates sperm adhesion to the oviduct reservoir using an in vitro model of oviduct epithelial spheroids (OES). Bovine oviducts from a local slaughterhouse were used to collect OF and isthmic fragments for the in vitro incubation of OES. Compared to a non-capacitating control medium, the pre-ovulatory OF significantly decreased by 80-90% the density of spermatozoa bound to OES without affecting sperm motility, membrane integrity, or sperm-cilia interactions. This effect on sperm binding was reproduced with (1) OF from different cycle stages and anatomical regions of the oviduct; (2) OF fractions of more than 3 kDa; (3) modified OF in which proteins were denatured or digested and (4) heparan sulphate but not hyaluronic acid, two glycosaminoglycans present in the OF. In conclusion, the OF significantly decreased the number of spermatozoa that bind to oviduct epithelial cells without affecting sperm motility and this effect was due to macromolecules, including heparan sulphate.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

