Cost Effectiveness of Different Platelet Preparation, Storage, Selection and Dosing Methods in Platelet Transfusion: A Systematic Review
- PMID: 37365482
- PMCID: PMC10471540
- DOI: 10.1007/s41669-023-00427-w
Cost Effectiveness of Different Platelet Preparation, Storage, Selection and Dosing Methods in Platelet Transfusion: A Systematic Review
Abstract
Background and objective: Evidence-based guidelines on platelet transfusion therapy assist clinicians to optimize patient care, but currently do not take into account costs associated with different methods used during the preparation, storage, selection and dosing of platelets for transfusion. This systematic review aimed to summarize the available literature regarding the cost effectiveness (CE) of these methods.
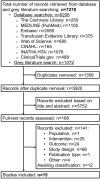
Methods: Eight databases and registries, as well as 58 grey literature sources, were searched up to 29 October 2021 for full economic evaluations comparing the CE of methods for preparation, storage, selection and dosing of allogeneic platelets intended for transfusion in adults. Incremental CE ratios, expressed as standardized cost (in 2022 EUR) per quality-adjusted life-year (QALY) or per health outcome, were synthesized narratively. Studies were critically appraised using the Philips checklist.
Results: Fifteen full economic evaluations were identified. Eight investigated the costs and health consequences (transfusion-related events, bacterial and viral infections or illnesses) of pathogen reduction. The estimated incremental cost per QALY varied widely from EUR 259,614 to EUR 36,688,323. For other methods, such as pathogen testing/culturing, use of apheresis instead of whole blood-derived platelets, and storage in platelet additive solution, evidence was sparse. Overall, the quality and applicability of the included studies was limited.
Conclusions: Our findings are of interest to decision makers who consider implementing pathogen reduction. For other preparation, storage, selection and dosing methods in platelet transfusion, CE remains unclear due to insufficient and outdated evaluations. Future high-quality research is needed to expand the evidence base and increase our confidence in the findings.
© 2023. The Author(s).
Conflict of interest statement
Relevant financial conflicts of interest directly related to this review: All authors declared not having any relevant direct financial conflict of interest. Relevant financial conflicts of interest not directly related to this review: JL, HVR, HS, BA, EDB, VC and PV are employees of Belgian Red Cross–Flanders, which is responsible for supplying adequate quantities of safe blood products to hospitals in Flanders and Brussels on a continuous basis and is funded by the Ministry of Social Affairs. Belgian Red Cross–Flanders received a grant from the European Blood Alliance to conduct this review. SS is an employee of NHS Blood and Transplant, which is the blood service supplier for England and manufactures platelets, and Chair International Collaboration for Transfusion Medicine Guidelines (ICTMG). SS reports research grant funding for trials using platelets and tranexamic acid. JG and SN declared not having any other financial conflicts of interest. NS received personal fees from the Canadian Blood Services for the International Collaboration for Transfusion Medicine Guidelines (ICTMG). SS received a grant award to conduct trials on use of platelets and tranexamic acid in patients with haematological cancer and a grant award to conduct trials on using platelets in neonates. Relevant intellectual conflicts of interest: JL, HVR, HS, BA, JG, EDB, VC and PV declared not having any intellectual conflict of interest. NS, SN and SS declare involvement in the ICTMG platelet guideline (SS is the chair, SN is the co-chair).
Figures
References
-
- FDA. Bacterial Risk control strategies for blood collection establishments and transfusion services to enhance the safety and availability of platelets for transfusion—guidance for Industry2020 December 2020.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources