Dopamine modulates the retinal clock through melanopsin-dependent regulation of cholinergic waves during development
- PMID: 37365544
- PMCID: PMC10294308
- DOI: 10.1186/s12915-023-01647-6
Dopamine modulates the retinal clock through melanopsin-dependent regulation of cholinergic waves during development
Abstract
Background: The mammalian retina contains an autonomous circadian clock that controls various aspects of retinal physiology and function, including dopamine (DA) release by amacrine cells. This neurotransmitter plays a critical role in retina development, visual signalling, and phase resetting of the retinal clock in adulthood. Interestingly, bidirectional regulation between dopaminergic cells and melanopsin-expressing retinal ganglion cells has been demonstrated in the adult and during development. Additionally, the adult melanopsin knockout mouse (Opn4 -/-) exhibits a shortening of the endogenous period of the retinal clock. However, whether DA and / or melanopsin influence the retinal clock mechanism during its maturation is still unknown.
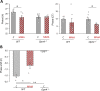
Results: Using wild-type Per2 Luc and melanopsin knockout (Opn4 -/-::Per2 Luc) mice at different postnatal stages, we found that the retina generates self-sustained circadian rhythms from postnatal day 5 in both genotypes and that the ability to express these rhythms emerges in the absence of external time cues. Intriguingly, only in wild-type explants, DA supplementation lengthened the endogenous period of the clock during the first week of postnatal development through both D1- and D2-like dopaminergic receptors. Furthermore, the blockade of spontaneous cholinergic retinal waves, which drive DA release in the early developmental stages, shortened the period and reduced the light-induced phase shift of the retinal clock only in wild-type retinas.
Conclusions: These data suggest that DA modulates the molecular core of the clock through melanopsin-dependent regulation of acetylcholine retinal waves, thus offering an unprecedented role of DA and melanopsin in the endogenous functioning and the light response of the retinal clock during development.
Keywords: Acetylcholine; Circadian rhythms; Dopamine; Light; Melanopsin; Retinal waves.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Hwang CK, Chaurasia SS, Jackson CR, Chan GC-K, Storm DR, Iuvone PM. Circadian Rhythm of Contrast Sensitivity Is Regulated by a Dopamine-Neuronal PAS-Domain Protein 2–Adenylyl Cyclase 1 Signaling Pathway in Retinal Ganglion Cells. J Neurosci. 2013;33:14989–97. doi: 10.1523/JNEUROSCI.2039-13.2013. - DOI - PMC - PubMed

