The safety of SGLT-2 inhibitors in diabetic patients submitted to elective percutaneous coronary intervention regarding kidney function: SAFE-PCI pilot study
- PMID: 37365618
- PMCID: PMC10291785
- DOI: 10.1186/s13098-023-01107-9
The safety of SGLT-2 inhibitors in diabetic patients submitted to elective percutaneous coronary intervention regarding kidney function: SAFE-PCI pilot study
Abstract
Background: Percutaneous coronary intervention (PCI) is one of the most performed well-succeeded therapeutic procedures worldwide, reducing symptoms and improving quality of life. Neutrophil Gelatinase-associated Lipocalin (NGAL) is a biomarker of acute kidney injury (AKI) produced early after an ischemic renal insult. Osmotic diuresis and the vasoconstriction of the afferent arteriole promoted by Sodium-glucose Cotransporter-2 Inhibitors (SGLT2i) generate a concern regarding the possibility of dehydration and consequent AKI. There is no consensus on the maintenance or discontinuation of SGTL2i in patients who will undergo PCI. This study aimed to evaluate the safety of empagliflozin in diabetic patients submitted to elective PCI regarding kidney function.
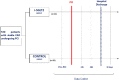
Methods: SAFE-PCI trial is a prospective, open-label, randomized (1:1), single-center pilot study and a follow-up of 30 days. The SGLT2i empagliflozin 25 mg daily was initiated at least 15 days before PCI in the intervention group and maintained until the end of the follow-up period. Serum NGAL was collected 6 h after PCI and creatinine before PCI, 24 h, and 48 h after the procedure. As per protocol, both groups received optimal medical treatment and standard protocol of nephroprotection.
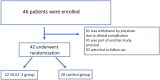
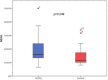
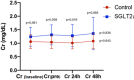
Results: A total of 42 patients were randomized (22 patients in the iSGLT-2 group and 20 patients in the control group). There was no difference between-group baseline data. The primary outcome (NGAL and creatinine values post PCI) did not differ in both groups: the mean NGAL value was 199 ng/dL in the empagliflozin group and 150 ng/dL in the control group (p = 0.249). Although there was an initial increase in creatinine in the SGLT-2i group compared to the control group between baseline creatinine and pre-PCI and 24 h post-PCI creatinine, no difference was detected in creatinine 48 h post-PCI (p = 0.065). The incidence of CI-AKI, determined by KDIGO criteria, in the iSGLT2-group was 13.6% and 10.0% in the control group without statistical difference.
Conclusion: The present study showed that the use of empagliflozin is safe regarding kidney function during elective PCI in patients with T2D when compared with no use of SGLT2i. Trial registration Our clinical study is registered on ClinicalTrials.gov with the following number: NCT05037695.
Keywords: Acute kidney injury; Contrast-induced nephropathy; Coronary artery disease; Percutaneous coronary intervention; SGLT2 inhibitors.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Feres F, et al. Guideline of the Brazilian society of cardiology and the brazilian society of hemodynamics and interventional cardiology on percutaneous coronary intervention. Braz Arch Cardiol. 2017;109(1):1–81.
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

