Homology-independent targeted insertion (HITI) enables guided CAR knock-in and efficient clinical scale CAR-T cell manufacturing
- PMID: 37365642
- PMCID: PMC10291796
- DOI: 10.1186/s12943-023-01799-7
Homology-independent targeted insertion (HITI) enables guided CAR knock-in and efficient clinical scale CAR-T cell manufacturing
Abstract
Background: Chimeric Antigen Receptor (CAR) T cells are now standard of care (SOC) for some patients with B cell and plasma cell malignancies and could disrupt the therapeutic landscape of solid tumors. However, access to CAR-T cells is not adequate to meet clinical needs, in part due to high cost and long lead times for manufacturing clinical grade virus. Non-viral site directed CAR integration can be accomplished using CRISPR/Cas9 and double-stranded DNA (dsDNA) or single-stranded DNA (ssDNA) via homology-directed repair (HDR), however yields with this approach have been limiting for clinical application (dsDNA) or access to large yields sufficient to meet the manufacturing demands outside early phase clinical trials is limited (ssDNA).
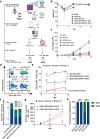
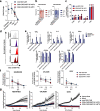
Methods: We applied homology-independent targeted insertion (HITI) or HDR using CRISPR/Cas9 and nanoplasmid DNA to insert an anti-GD2 CAR into the T cell receptor alpha constant (TRAC) locus and compared both targeted insertion strategies in our system. Next, we optimized post-HITI CRISPR EnrichMENT (CEMENT) to seamlessly integrate it into a 14-day process and compared our knock-in with viral transduced anti-GD2 CAR-T cells. Finally, we explored the off-target genomic toxicity of our genomic engineering approach.
Results: Here, we show that site directed CAR integration utilizing nanoplasmid DNA delivered via HITI provides high cell yields and highly functional cells. CEMENT enriched CAR T cells to approximately 80% purity, resulting in therapeutically relevant dose ranges of 5.5 × 108-3.6 × 109 CAR + T cells. CRISPR knock-in CAR-T cells were functionally comparable with viral transduced anti-GD2 CAR-T cells and did not show any evidence of off-target genomic toxicity.
Conclusions: Our work provides a novel platform to perform guided CAR insertion into primary human T-cells using nanoplasmid DNA and holds the potential to increase access to CAR-T cell therapies.
Keywords: CRISPR/Cas9; GMP; Genomic safety; Homology-independent targeted insertion (HITI); Non-viral CAR-T cell; Targeted insertion.
© 2023. The Author(s).
Conflict of interest statement
H.B.W., C.L.M. and S.A.F. are inventors on a Stanford University provisional patent application pending on Homology-Independent Targeted DNA Insertion in human T cells. A.G.M. has been employed by Maxcyte during this study. G.L.K. is an employee of Integrated DNA Technologies, which sells reagents described in this manuscript. G.L.K owns equity in Danaher Corporation, which is the parent company of Integrated DNA Technologies. Shabnum Patel is an employee of Cargo Therapeutics. E.S. consults for Lepton Pharmaceuticals and Galaria; and holds equity in Lyell Immunopharma. C.L.M. holds patents focused on CAR T cells therapies, is a cofounder and holds equity in Lyell Immunopharma, CARGO Therapeutics and Link Cell Therapies, which are developing CAR-based therapies, and consults for Lyell, CARGO, Link, Apricity, Nektar, Immatics, Mammoth and Ensoma.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

