Effects of Platelet-Rich Plasma on the Oxymetholone-Induced Testicular Toxicity
- PMID: 37366872
- PMCID: PMC10297451
- DOI: 10.3390/diseases11020084
Effects of Platelet-Rich Plasma on the Oxymetholone-Induced Testicular Toxicity
Abstract
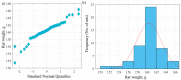
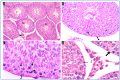
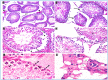
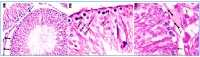
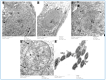
Oxymetholone is one of the anabolic steroids that has widely been used among teenagers and athletes to increase their muscle bulk. It has undesirable effects on male health and fertility. In this study, the therapeutic effects of platelet-rich plasma (PRP) on oxymetholone-induced testicular toxicity were investigated in adult albino rats. During the experiments, 49 adult male albino rats were divided into 4 main groups: Group 0 (donor group) included 10 rats for the donation of PRP, Group I (control group) included 15 rats, Group II included 8 rats that received 10 mg/kg of oxymetholone orally, once daily, for 30 days, and Group III included 16 rats and was subdivided into 2 subgroups (IIIa and IIIb) that received oxymetholone the same as group II and then received PRP once and twice, respectively. Testicular tissues of all examined rats were obtained for processing and histological examination and sperm smears were stained and examined for sperm morphology. Oxymetholone-treated rats revealed wide spaces in between the tubules, vacuolated cytoplasm, and dark pyknotic nuclei of most cells, as well as deposition of homogenous acidophilic material between the tubules. Electron microscopic examination showed vacuolated cytoplasm of most cells, swollen mitochondria, and perinuclear dilatation. Concerning subgroup IIIa (PRP once), there was a partial improvement in the form of decreased vacuolations and regeneration of spermatogenic cells, as well as a reasonable improvement in sperm morphology. Regarding subgroup IIIb (PRP twice), histological sections revealed restoration of the normal testicular structure to a great extent, regeneration of the spermatogenic cells, and most sperms had normal morphology. Thus, it is recommended to use PRP to minimize structural changes in the testis of adult albino rats caused by oxymetholone.
Keywords: albino rat; histology investigation; oxymetholone; platelet-rich plasma; testis; therapeutic effects.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Wang Y., Bai L., Li H., Yang W., Li M. Protective Effects of Lepidium draba L. Leaves Extract on Testis Histopathology, Oxidative Stress Indicators, Serum Reproductive Hormones and Inflammatory Signalling in Oxymetholone-treated Rat. Andrologia. 2021;53:e14239. doi: 10.1111/and.14239. - DOI - PubMed
-
- Mehrpour O., Nakhaee S., Barangi S., Karimi G.B.T.-R.M. Reference Module in Biomedical Sciences. Elsevier; Amsterdam, The Netherlands: 2022.
-
- Akbari Bazm M., Goodarzi N., Shahrokhi S.R., Khazaei M. The Effects of Hydroalcoholic Extract of Vaccinium arctostaphylos L. on Sperm Parameters, Oxidative Injury and Apoptotic Changes in Oxymetholone-induced Testicular Toxicity in Mouse. Andrologia. 2020;52:e13522. doi: 10.1111/and.13522. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

