A Systematic Review and Meta-Analysis of Trifluridine/Tipiracil plus Bevacizumab for the Treatment of Metastatic Colorectal Cancer: Evidence from Real-World Series
- PMID: 37366880
- PMCID: PMC10297708
- DOI: 10.3390/curroncol30060397
A Systematic Review and Meta-Analysis of Trifluridine/Tipiracil plus Bevacizumab for the Treatment of Metastatic Colorectal Cancer: Evidence from Real-World Series
Abstract
Background: Colorectal cancer is the most prevalent gastrointestinal neoplasm. When metastatic, the disease has limited systemic treatment options. Novel targeted therapies have expanded these options for subsets with specific molecular alterations, such as microsatellite instability (MSI)-high cancers, but additional treatments and combinations are in urgent need to improve outcomes and improve survival of this incurable disease. The fluoropyrimidine-derivative trifluridine, in combination with tipiracil, has been introduced as a third-line treatment, and more recently, it was studied in combination with bevacizumab. This meta-analysis reports on studies with this combination in clinical practice outside clinical trials.
Methods: A literature search in the Medline/PubMed and Embase databases was executed for finding series of trifluridine/tipiracil with bevacizumab in metastatic colorectal cancer. Criteria for inclusion in the meta-analysis were English or French language of the report, inclusion of twenty or more patients with metastatic colorectal cancer treated with trifluridine/tipiracil in combination with bevacizumab outside of a trial and containing information regarding response rates, progression-free survival (PFS), and overall survival (OS). Information on the demographics of the patients and on adverse effects of treatment was also collected.
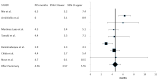
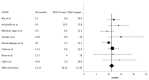
Results: Eight series with a total of 437 patients were eligible for the meta-analysis. The performed meta-analysis discovered a summary response rate (RR) of 2.71% (95% confidence interval (CI): 1.11-4.32%) and a disease control rate (DCR) of 59.63% (95% CI: 52.06-67.21%). Summary PFS was 4.56 months (95% CI: 3.57-5.55 months), and summary OS was 11.17 months (95% CI: 10.15-12.19 months). Common adverse effects identified mirrored the adverse-effect profile of the two components of the combination.
Conclusion: The current systematic review and meta-analysis reports the efficacy of trifluridine/tipiracil with bevacizumab in advanced lines of therapy for metastatic colorectal cancer in the setting of clinical practice outside clinical trials. Discovery of predictive biomarkers of response to trifluridine/tipiracil with bevacizumab will promote the tailoring of this treatment to individual patients to maximize clinical benefit.
Keywords: TAS-102; anti-angiogenic; colorectal cancer; later line; metastatic.
Conflict of interest statement
The author declares no conflict of interest.
Figures



Similar articles
-
Epidermal growth factor receptor (EGFR) inhibitors for metastatic colorectal cancer.Cochrane Database Syst Rev. 2017 Jun 27;6(6):CD007047. doi: 10.1002/14651858.CD007047.pub2. Cochrane Database Syst Rev. 2017. PMID: 28654140 Free PMC article.
-
Targeted therapy combined with immunotherapy vs trifluridine/tipiracil with bevacizumab as late-line therapy in metastatic colorectal cancer.World J Gastroenterol. 2025 Aug 7;31(29):109947. doi: 10.3748/wjg.v31.i29.109947. World J Gastroenterol. 2025. PMID: 40809924 Free PMC article.
-
Trifluridine-tipiracil plus bevacizumab versus trifluridine-tipiracil monotherapy for chemorefractory metastatic colorectal cancer: a systematic review and meta-analysis.BMC Cancer. 2024 Jun 3;24(1):674. doi: 10.1186/s12885-024-12447-8. BMC Cancer. 2024. PMID: 38825703 Free PMC article.
-
Trifluridine-Tipiracil and Bevacizumab in Refractory Metastatic Colorectal Cancer.N Engl J Med. 2023 May 4;388(18):1657-1667. doi: 10.1056/NEJMoa2214963. N Engl J Med. 2023. PMID: 37133585 Clinical Trial.
-
Vascular-endothelial-growth-factor (VEGF) targeting therapies for endocrine refractory or resistant metastatic breast cancer.Cochrane Database Syst Rev. 2012 Jul 11;2012(7):CD008941. doi: 10.1002/14651858.CD008941.pub2. Cochrane Database Syst Rev. 2012. PMID: 22786517 Free PMC article.
Cited by
-
Cost-effectiveness analysis of fruquintinib in Chinese patients with refractory metastatic colorectal cancer.Int J Clin Pharm. 2024 Aug;46(4):872-880. doi: 10.1007/s11096-024-01721-1. Epub 2024 Apr 20. Int J Clin Pharm. 2024. PMID: 38642249
-
Advances in Targeted and Chemotherapeutic Strategies for Colorectal Cancer: Current Insights and Future Directions.Biomedicines. 2025 Mar 5;13(3):642. doi: 10.3390/biomedicines13030642. Biomedicines. 2025. PMID: 40149618 Free PMC article. Review.
-
HER2-related biomarkers predict clinical outcomes with trastuzumab deruxtecan treatment in patients with HER2-expressing metastatic colorectal cancer: biomarker analyses of DESTINY-CRC01.Nat Commun. 2024 Nov 25;15(1):10213. doi: 10.1038/s41467-024-53223-3. Nat Commun. 2024. PMID: 39587050 Free PMC article. Clinical Trial.
-
Transarterial chemoembolisation with irinotecan (irinotecan-TACE) as salvage or post-inductive therapy for colorectal cancer liver metastases: effectiveness results from the CIREL study.ESMO Open. 2025 Mar;10(3):104292. doi: 10.1016/j.esmoop.2025.104292. Epub 2025 Feb 15. ESMO Open. 2025. PMID: 39954388 Free PMC article.
-
Survival Outcomes with Regorafenib and/or Trifluridine/Tipiracil Sequencing to Rechallenge with Third-Line Regimens in Metastatic Colorectal Cancer: A Multicenter Retrospective Real-World Subgroup Comparison from the ReTrITA Study.Curr Oncol. 2024 Dec 4;31(12):7793-7808. doi: 10.3390/curroncol31120574. Curr Oncol. 2024. PMID: 39727697 Free PMC article.
References
-
- Rankin A., Klempner S.J., Erlich R., Sun J.X., Grothey A., Fakih M., George T.J., Jr., Lee J., Ross J.S., Stephens P.J., et al. Broad Detection of Alterations Predicted to Confer Lack of Benefit from EGFR Antibodies or Sensitivity to Targeted Therapy in Advanced Colorectal Cancer. Oncologist. 2016;21:1306–1314. doi: 10.1634/theoncologist.2016-0148. - DOI - PMC - PubMed
-
- Tabernero J., Grothey A., Van Cutsem E., Yaeger R., Wasan H., Yoshino T., Desai J., Ciardiello F., Loupakis F., Hong Y.S., et al. Encorafenib Plus Cetuximab as a New Standard of Care for Previously Treated BRAF V600E-Mutant Metastatic Colorectal Cancer: Updated Survival Results and Subgroup Analyses from the BEACON Study. J. Clin. Oncol. 2021;39:273–284. doi: 10.1200/JCO.20.02088. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

