HR+/HER2- Advanced Breast Cancer Treatment in the First-Line Setting: Expert Review
- PMID: 37366894
- PMCID: PMC10297170
- DOI: 10.3390/curroncol30060411
HR+/HER2- Advanced Breast Cancer Treatment in the First-Line Setting: Expert Review
Abstract
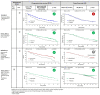
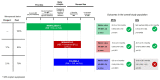
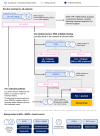
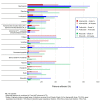
The approval of CDK4/6 inhibitors has dramatically improved care for the treatment of HR+/HER2- advanced breast cancer, but navigating the rapidly-expanding treatment evidence base is challenging. In this narrative review, we provide best-practice recommendations for the first-line treatment of HR+/HER2- advanced breast cancer in Canada based on relevant literature, clinical guidelines, and our own clinical experience. Due to statistically significant improvements in overall survival and progression-free survival, ribociclib + aromatase inhibitor is our preferred first-line treatment for de novo advanced disease or relapse ≥12 months after completion of adjuvant endocrine therapy and ribociclib or abemaciclib + fulvestrant is our preferred first-line treatment for patients experiencing early relapse. Abemaciclib or palbociclib may be used when alternatives to ribociclib are needed, and endocrine therapy can be used alone in the case of contraindication to CDK4/6 inhibitors or limited life expectancy. Considerations for special populations-including frail and fit elderly patients, as well as those with visceral disease, brain metastases, and oligometastatic disease-are also explored. For monitoring, we recommend an approach across CDK4/6 inhibitors. For mutational testing, we recommend routinely performing ER/PR/HER2 testing to confirm the subtype of advanced disease at the time of progression and to consider ESR1 and PIK3CA testing for select patients. Where possible, engage a multidisciplinary care team to apply evidence in a patient-centric manner.
Keywords: CDK4/6 inhibitors; HR+/HER2–; advanced breast cancer; mutational testing; treatment considerations.
Conflict of interest statement
K.J.J.: Advisory boards—Amgen, AstraZeneca, Apo Biologix, Eli Lilly, Esai, Genomic Health, Gilead Sciences, Knight Therapeutics, Merck, Myriad Genetics Inc, Pfizer, Roche, Seagen, Novartis, Viatris. Speaker honoraria—Amgen, AstraZeneca, Apo Biologix, Eli Lilly, Esai, Genomic Health, Gilead Sciences, Knight Therapeutics, Merck, Myriad Genetics Inc, Pfizer, Roche, Seagen, Novartis, Viatris. Grants/research support—AstraZeneca, Eli Lilly, Seagen; N.B.: Honoraria—Merck, Novartis, Astra Zeneca; C.B.-M.: Advisory boards/consultancy and speaker honoraria*—Astra Zeneca, Agendia, BMS, Knight, Merck, Lilly*, Novartis*, Pfizer*, Roche, Seagen, Taiho, Sanofi, Mylan, Gilead; S.E.: Apobiologix, Astellas, Astra Zeneca, Bayer, Eli Lilly, Gilead; K.G.: Advisory boards—Astra Zeneca, Merck, Gilead, Seagan, Pfizer, Novartis, Lilly; J.-W.H.: Advisory boards and speaker honoraria—Astra Zeneca, Novartis, Pfizer, Gilead, Eli Lilly, Seagen, Merck; J.F.H.: TBD; S.S.: Advisory board and speaker honoraria—Astra Zeneca, Novartis.
Figures
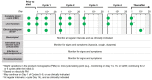



References
-
- CCS Breast Cancer Statistics. [(accessed on 1 May 2022)]. Available online: https://cancer.ca/en/cancer-information/cancer-types/breast/statistics.
-
- Robertson J.F.R., Bondarenko I.M., Trishkina E., Dvorkin M., Panasci L., Manikhas A., Shparyk Y., Cardona-Huerta S., Cheung K.-L., Philco-Salas M.J., et al. Fulvestrant 500 Mg versus Anastrozole 1 Mg for Hormone Receptor-Positive Advanced Breast Cancer (FALCON): An International, Randomised, Double-Blind, Phase 3 Trial. Lancet. 2016;388:2997–3005. doi: 10.1016/S0140-6736(16)32389-3. - DOI - PubMed
-
- Mehta R.S., Barlow W.E., Albain K.S., Vandenberg T.A., Dakhil S.R., Tirumali N.R., Lew D.L., Hayes D.F., Gralow J.R., Linden H.M., et al. Overall Survival with Fulvestrant plus Anastrozole in Metastatic Breast Cancer. N. Eng. J. Med. 2019;380:1226–1234. doi: 10.1056/NEJMoa1811714. - DOI - PMC - PubMed
-
- Piccart M., Hortobagyi G.N., Campone M., Pritchard K.I., Lebrun F., Ito Y., Noguchi S., Perez A., Rugo H.S., Deleu I., et al. Everolimus plus Exemestane for Hormone-Receptor-Positive, Human Epidermal Growth Factor Receptor-2-Negative Advanced Breast Cancer: Overall Survival Results from BOLERO-2. Ann. Oncol. 2014;25:2357–2362. doi: 10.1093/annonc/mdu456. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

