Treatment Settings and Outcomes with Regorafenib and Trifluridine/Tipiracil at Third-Line Treatment and beyond in Metastatic Colorectal Cancer: A Real-World Multicenter Retrospective Study
- PMID: 37366896
- PMCID: PMC10296859
- DOI: 10.3390/curroncol30060413
Treatment Settings and Outcomes with Regorafenib and Trifluridine/Tipiracil at Third-Line Treatment and beyond in Metastatic Colorectal Cancer: A Real-World Multicenter Retrospective Study
Abstract
Background: Patients with refractory mCRC rarely undergo third-line or subsequent treatment. This strategy could negatively impact their survival. In this setting, regorafenib (R) and trifluridine/tipiracil (T) are two key new treatment options with statistically significant improvements in overall survival (OS), progression-free survival (PFS), and disease control with different tolerance profiles. This study aimed to retrospectively evaluate the efficacy and safety profiles of these agents in real-world practice.
Materials and methods: In 2012-2022, 866 patients diagnosed with mCRC who received sequential R and T (T/R, n = 146; R/T, n = 116]) or T (n = 325]) or R (n = 279) only were retrospectively recruited from 13 Italian cancer institutes.
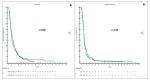
Results: The median OS is significantly longer in the R/T group (15.9 months) than in the T/R group (13.9 months) (p = 0.0194). The R/T sequence had a statistically significant advantage in the mPFS, which was 8.8 months with T/R vs. 11.2 months with R/T (p = 0.0005). We did not find significant differences in outcomes between groups receiving T or R only. A total of 582 grade 3/4 toxicities were recorded. The frequency of grade 3/4 hand-foot skin reactions was higher in the R/T sequence compared to the reverse sequence (37.3% vs. 7.4%) (p = 0.01), while grade 3/4 neutropenia was slightly lower in the R/T group than in the T/R group (66.2% vs. 78.2%) (p = 0.13). Toxicities in the non-sequential groups were similar and in line with previous studies.
Conclusions: The R/T sequence resulted in a significantly longer OS and PFS and improved disease control compared with the reverse sequence. R and T given not sequentially have similar impacts on survival. More data are needed to define the best sequence and to explore the efficacy of sequential (T/R or R/T) treatment combined with molecular-targeted drugs.
Keywords: metastatic colorectal cancer; real-world study; regorafenib; third-line therapy; trifluridine/tipiracil.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®®) Colon Cancer Version 3.2022–January 25, 2023. [(accessed on 25 January 2023)]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
-
- Van Cutsem E., Cervantes A., Adam R., Sobrero A., Van Krieken J.H., Aderka D., Aranda Aguilar E., Bardelli A., Benson A., Bodoky G., et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

