Prediction of Disease Progression to Upfront Pembrolizumab Monotherapy in Advanced Non-Small-Cell Lung Cancer with High PD-L1 Expression Using Baseline CT Disease Quantification and Smoking Pack Years
- PMID: 37366902
- PMCID: PMC10297400
- DOI: 10.3390/curroncol30060419
Prediction of Disease Progression to Upfront Pembrolizumab Monotherapy in Advanced Non-Small-Cell Lung Cancer with High PD-L1 Expression Using Baseline CT Disease Quantification and Smoking Pack Years
Abstract
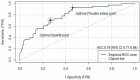
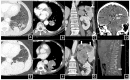
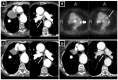
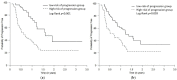
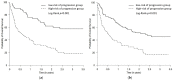
Health Canada approved pembrolizumab in the first-line setting for advanced non-small-cell lung cancer with PD-L1 ≥ 50% and no EGFR/ALK aberration. The keynote 024 trial showed 55% of such patients progress with pembrolizumab monotherapy. We propose that the combination of baseline CT and clinical factors can help identify those patients who may progress. In 138 eligible patients from our institution, we retrospectively collected their baseline variables, including baseline CT findings (primary lung tumor size and metastatic site), smoking pack years, performance status, tumor pathology, and demographics. The treatment response was assessed via RECIST 1.1 using the baseline and first follow-up CT. Associations between the baseline variables and progressive disease (PD) were tested by logistic regression analyses. The results showed 46/138 patients had PD. The baseline CT "number of involved organs" by metastasis and smoking pack years were independently associated with PD (p < 0.05), and the ROC analysis showed a good performance of the model that integrated these variables in predicting PD (AUC: 0.79). This pilot study suggests that the combination of baseline CT disease and smoking PY can identify who may progress on pembrolizumab monotherapy and can potentially facilitate decision-making for the optimal first-line treatment in the high PD-L1 cohort.
Keywords: PDL1; baseline CT; non-small-cell lung cancer; pembrolizumab; smoking pack years; survival; tumor response.
Conflict of interest statement
A.S. reports no competing interest. C.H. reports grants from AstraZeneca, EMD Serono, and Roche and receives honoraria from Abbvie, Amgen, Astra Zeneca, Bayer, BMS, Eisai, EMD Serono, Janssen, Jazz, Merck, Novartis, Pfizer, Roche and Takeda. Q.Y. reports no competing interests. J.Z. reports grants from Merck; grants and personal fees from Johnson and Johnson and Novartis; and personal fees from Bristol Myers Squibb, AstraZeneca, GenePlus, Innovent, and Hengrui outside the submitted work. I.J., J.L., M.M., L.W., Y.W., S.L. and C.M. report no competing interests. B.M. receives honoraria and participates in advisory boards for Astra Zeneca, BMS, Merck, Roche, Jazz, Pfizer, Jansen, Roche, and Novartis. R.Y. reports no competing interests.
Figures
References
-
- Canadian Cancer Statistics Advisory Committee in Collaboration with the Canadian Cancer Society, Statistics Canada and the Public Health Agency of Canada. Canadian Cancer Statistics 2021. Toronto, ON: Canadian Cancer Society. 2021. [(accessed on 5 June 2023)]. Available online: https://www.cancer.ca/Canadian-Cancer-Statistics-2021-EN.
-
- Mok T.S.K., Wu Y.L., Kudaba I., Kowalski D.M., Cho B.C., Turna H.Z., Castro G., Jr., Srimuninnimit V., Laktionov K.K., Bondarenko I., et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

