Systematic Review of Calcineurin Inhibitors and Incidence of Skin Malignancies after Kidney Transplantation in Adult Patients: A Study of 309,551 Cases
- PMID: 37366913
- PMCID: PMC10296938
- DOI: 10.3390/curroncol30060430
Systematic Review of Calcineurin Inhibitors and Incidence of Skin Malignancies after Kidney Transplantation in Adult Patients: A Study of 309,551 Cases
Abstract
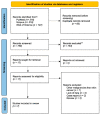
The purpose of this systematic review and meta-analysis was to compare the risk of non-melanoma skin cancer (NMSC) and melanoma development in renal transplant recipients who receive calcineurin inhibitors to that of patients treated with other immunosuppressive agents, and investigate the possible association between the type of maintenance immunosuppression and the incidence of NSMC and melanoma in this group of patients. The authors searched databases such as PubMed, Scopus, and Web of Science for articles that would help establish the influence of calcineurin inhibitors on skin cancer development. The inclusion criteria for the study consisted of randomized clinical trials, cohort studies, and case-control studies that compared patients who received kidney transplants and were treated with a calcineurin inhibitor (CNI), such as cyclosporine A (CsA) or tacrolimus (Tac), to those who received alternative immunosuppressants and did not receive a CNI. Seven articles were analyzed overall. The results revealed a correlation between CNI treatment in renal transplant recipients and increased total skin cancer risk (OR 1.28; 95% CI: 0.10-16.28; p < 0.01), melanoma risk (OR 1.09; 95% CI: 0.25-4.74; p < 0.01), and NMSC risk (OR 1.16; 95% CI: 0.41-3.26; p < 0.01). In conclusion, the calcineurin inhibitors used after kidney transplantation are associated with a higher risk of skin cancer-both non-melanoma and melanoma-when compared with other immunosuppressive therapies. This finding suggests that careful monitoring for skin lesions in post-transplant patients must be conducted. However, the decision on the kind of immunotherapy used should always be considered on an individual basis for each renal transplant recipient.
Keywords: calcineurin inhibitors; cyclosporine; melanoma skin cancer; meta-analysis; non-melanoma skin cancer; renal transplant recipients; renal transplantation; skin cancer; tacrolimus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
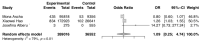
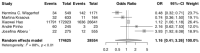
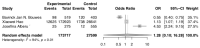

Similar articles
-
Polyclonal and monoclonal antibodies for induction therapy in kidney transplant recipients.Cochrane Database Syst Rev. 2017 Jan 11;1(1):CD004759. doi: 10.1002/14651858.CD004759.pub2. Cochrane Database Syst Rev. 2017. PMID: 28073178 Free PMC article.
-
CMV and BKPyV Infections in Renal Transplant Recipients Receiving an mTOR Inhibitor-Based Regimen Versus a CNI-Based Regimen: A Systematic Review and Meta-Analysis of Randomized, Controlled Trials.Clin J Am Soc Nephrol. 2017 Aug 7;12(8):1321-1336. doi: 10.2215/CJN.13221216. Epub 2017 Jun 2. Clin J Am Soc Nephrol. 2017. PMID: 28576905 Free PMC article.
-
Calcineurin inhibitor minimisation versus continuation of calcineurin inhibitor treatment for liver transplant recipients.Cochrane Database Syst Rev. 2012 Mar 14;2012(3):CD008852. doi: 10.1002/14651858.CD008852.pub2. Cochrane Database Syst Rev. 2012. PMID: 22419339 Free PMC article.
-
Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study.Health Technol Assess. 2005 May;9(21):1-179, iii-iv. doi: 10.3310/hta9210. Health Technol Assess. 2005. PMID: 15899149
-
Immunosuppressive treatment for primary membranous nephropathy in adults with nephrotic syndrome.Cochrane Database Syst Rev. 2021 Nov 15;11(11):CD004293. doi: 10.1002/14651858.CD004293.pub4. Cochrane Database Syst Rev. 2021. PMID: 34778952 Free PMC article.
Cited by
-
Rapamycin inhibits B16 melanoma cell viability invitro and invivo by inducing autophagy and inhibiting the mTOR/p70‑S6k pathway.Oncol Lett. 2024 Feb 2;27(4):140. doi: 10.3892/ol.2024.14273. eCollection 2024 Apr. Oncol Lett. 2024. PMID: 38385108 Free PMC article.
-
Belatacept and non-melanoma skin cancer risk in kidney transplant recipients: a narrative review from a mechanistic and clinical perspective.BMC Nephrol. 2025 Aug 23;26(1):487. doi: 10.1186/s12882-025-04373-z. BMC Nephrol. 2025. PMID: 40849621 Review.
-
Cutaneous Squamous Cell Carcinoma in Patients with Solid-Organ-Transplant-Associated Immunosuppression.Cancers (Basel). 2024 Sep 4;16(17):3083. doi: 10.3390/cancers16173083. Cancers (Basel). 2024. PMID: 39272941 Free PMC article. Review.
-
Basal Cell Carcinoma Arising in a Previous Full-Thickness Graft Donor Site: A Case Report and Comprehensive Literature Review.J Clin Med. 2025 Jan 17;14(2):591. doi: 10.3390/jcm14020591. J Clin Med. 2025. PMID: 39860596 Free PMC article. Review.
-
International incidence of melanoma in heart transplant recipients: a meta-analysis.Melanoma Res. 2025 Feb 1;35(1):24-30. doi: 10.1097/CMR.0000000000001008. Epub 2024 Oct 7. Melanoma Res. 2025. PMID: 39365850 Free PMC article.
References
-
- Haberal M., Boyvat F., Akdur A., Kırnap M., Özçelik Ü., Yarbuğ Karakayalı F. Surgical complications after kidney transplantation. Exp. Clin. Transpl. 2016;14:587–595. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

