2023 Canadian Colposcopy Guideline: A Risk-Based Approach to Management and Surveillance of Cervical Dysplasia
- PMID: 37366914
- PMCID: PMC10297713
- DOI: 10.3390/curroncol30060431
2023 Canadian Colposcopy Guideline: A Risk-Based Approach to Management and Surveillance of Cervical Dysplasia
Abstract
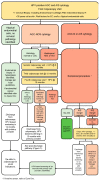
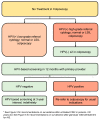
This guideline provides evidence-based guidance on the risk-based management of cervical dysplasia in the colposcopy setting in the context of primary HPV-based screening and HPV testing in colposcopy. Colposcopy management of special populations is also discussed. The guideline was developed by a working group in collaboration with the Gynecologic Oncology Society of Canada (GOC), Society of Colposcopists of Canada (SCC) and the Canadian Partnership Against Cancer (CPAC). The literature informing these guidelines was obtained through a systematic review of the relevant literature via a multi-step search process led by information specialists. The literature was reviewed up to June 2021 with manual searches of relevant national guidelines and more recent publications. Quality of the evidence and strength of recommendations was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework. The intended users of this guideline include gynecologists, colposcopists, screening programs and healthcare facilities. Implementation of the recommendations is intended to promote equitable and standardized care for all people undergoing colposcopy in Canada. The risk-based approach aims to improve personalized care and reduce over-/under-treatment in colposcopy.
Keywords: HPV; cervical cancer; colposcopy; guideline; human papillomavirus.
Conflict of interest statement
A.S. is the current president of the Society of Canadian Colposcopists. K.W. is the president-elect of the Society of Canadian Colposcopists. K.W. has received speaker honoraria from Merck Canada. M.-H.A. has received consultation fees from GSK and Merck Canada. J.B. has received honoraria from GSK and Merck Canada and research support from the Canadian Cancer Society. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Canadian Guideline on the Management of a Positive Human Papillomavirus Test and Guidance for Specific Populations.Curr Oncol. 2023 Jun 9;30(6):5652-5679. doi: 10.3390/curroncol30060425. Curr Oncol. 2023. PMID: 37366908 Free PMC article.
-
[Health technology assessment report: HPV DNA based primary screening for cervical cancer precursors].Epidemiol Prev. 2012 May-Aug;36(3-4 Suppl 1):e1-72. Epidemiol Prev. 2012. PMID: 22828243 Italian.
-
[Real-world research on cervical cancer screening program and effect evaluation for Chinese population].Zhonghua Zhong Liu Za Zhi. 2018 Oct 23;40(10):764-771. doi: 10.3760/cma.j.issn.0253-3766.2018.10.008. Zhonghua Zhong Liu Za Zhi. 2018. PMID: 30392341 Chinese.
-
Cervical Colposcopy: Indications and Risk Assessment.Am Fam Physician. 2020 Jul 1;102(1):39-48. Am Fam Physician. 2020. PMID: 32603071
-
Clinical Practice Guidelines on the Screening and Treatment of Precancerous Lesions for Cervical Cancer Prevention in Saudi Arabia.Ann Saudi Med. 2016 Sep-Oct;36(5):313-320. doi: 10.5144/0256-4947.2016.313. Ann Saudi Med. 2016. PMID: 27710981 Free PMC article. Review.
Cited by
-
Development of a clinical prediction model for pathological upgrading in low-grade squamous intraepithelial lesions following cervical conization.Cytojournal. 2024 Oct 11;21:37. doi: 10.25259/Cytojournal_7_2024. eCollection 2024. Cytojournal. 2024. PMID: 39563666 Free PMC article.
-
Will methylation assays be part of a full molecular strategy to improve clinical management of cervical neoplasia?Epigenomics. 2024;16(18):1197-1201. doi: 10.1080/17501911.2024.2402684. Epub 2024 Sep 25. Epigenomics. 2024. PMID: 39320908 No abstract available.
-
Clinical Outcomes of Cervical Adenocarcinoma In Situ According to Conservative or Demolitive Treatment: A Systematic Review and Meta-Analysis.Cancers (Basel). 2025 May 30;17(11):1839. doi: 10.3390/cancers17111839. Cancers (Basel). 2025. PMID: 40507318 Free PMC article. Review.
-
The Relationship Between Cervicovaginal Infection, Human Papillomavirus Infection and Cervical Intraepithelial Neoplasia in Romanian Women.Diseases. 2025 Jan 16;13(1):18. doi: 10.3390/diseases13010018. Diseases. 2025. PMID: 39851482 Free PMC article.
-
Screening for Cervical Cancer in Pregnancy.Oncol Rev. 2023 Oct 17;17:11429. doi: 10.3389/or.2023.11429. eCollection 2023. Oncol Rev. 2023. PMID: 37915464 Free PMC article. Review.
References
-
- Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. World Health Organization; Geneva, Switzerland: 2021. [(accessed on 4 November 2022)]. Available online: www.wipo.int/amc/en/mediation/rules/
-
- Brenner D. Canadian Cancer Statistics Advisory Committee in Collaboration with the Canadian Cancer Society, Statistics Canada and the Public Health Agency of Canada. “Canadian Cancer Statistics 2021”. Canadian Cancer Society; Toronto, ON, Canada: 2021.
-
- Action Plan for the Elimination of cervical cancer in Canada 2020–2030. 2020. [(accessed on 4 November 2022)]. Available online: https://www.partnershipagainstcancer.ca/topics/elimination-cervical-canc...
-
- Zigras T., Mayrand M.-H., Bouchard C., Salvador S., Eiriksson L., Almadin C., Kean S., Dean E., Malhotra U., Todd N., et al. Canadian Guideline on the Management of a Positive Human Papillomavirus Test and Guidance for Specific Populations. Curr. Oncol. 2023;30:5652–5679. doi: 10.3390/curroncol30060425. - DOI - PMC - PubMed
-
- Perkins R.B., Guido R.S., Castle P.E., Chelmow D., Einstein M.H., Garcia F., Huh W.K., Kim J.J., Moscicki A.-B., Nayar R., et al. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J. Low. Genit. Tract Dis. 2020;24:102–131. doi: 10.1097/LGT.0000000000000525. - DOI - PMC - PubMed

