Can Molecular Biomarkers Help Reduce the Overtreatment of DCIS?
- PMID: 37366916
- PMCID: PMC10297044
- DOI: 10.3390/curroncol30060433
Can Molecular Biomarkers Help Reduce the Overtreatment of DCIS?
Abstract
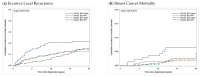
Ductal carcinoma in situ (DCIS), especially in the era of mammographic screening, is a commonly diagnosed breast tumor. Despite the low breast cancer mortality risk, management with breast conserving surgery (BCS) and radiotherapy (RT) is the prevailing treatment approach in order to reduce the risk of local recurrence (LR), including invasive LR, which carries a subsequent risk of breast cancer mortality. However, reliable and accurate individual risk prediction remains elusive and RT continues to be standardly recommended for most women with DCIS. Three molecular biomarkers have been studied to better estimate LR risk after BCS-Oncotype DX DCIS score, DCISionRT Decision Score and its associated Residual Risk subtypes, and Oncotype 21-gene Recurrence Score. All these molecular biomarkers represent important efforts towards improving predicted risk of LR after BCS. To prove clinical utility, these biomarkers require careful predictive modeling with calibration and external validation, and evidence of benefit to patients; on this front, further research is needed. Most trials do not incorporate molecular biomarkers in evaluating de-escalation of therapy for DCIS; however, one-the Prospective Evaluation of Breast-Conserving Surgery Alone in Low-Risk DCIS (ELISA) trial-incorporates the Oncotype DX DCIS score in defining a low-risk population and is an important next step in this line of research.
Keywords: DCIS; biomarkers; ductal carcinoma in situ.
Conflict of interest statement
E.R. has received research grant funding from Genomic Health Inc., outside of the submitted work. All other authors declare no conflict of interest.
Figures
Similar articles
-
Molecular Evaluation of Breast Ductal Carcinoma in Situ with Oncotype DX DCIS.Am J Pathol. 2019 May;189(5):975-980. doi: 10.1016/j.ajpath.2018.12.003. Epub 2018 Dec 31. Am J Pathol. 2019. PMID: 30605628 Review.
-
DCIS: Risk Assessment in the Molecular Era.Semin Radiat Oncol. 2022 Jul;32(3):189-197. doi: 10.1016/j.semradonc.2022.01.005. Semin Radiat Oncol. 2022. PMID: 35688517 Review.
-
Multigene Expression Assay and Benefit of Radiotherapy After Breast Conservation in Ductal Carcinoma in Situ.J Natl Cancer Inst. 2017 Apr 1;109(4):djw256. doi: 10.1093/jnci/djw256. J Natl Cancer Inst. 2017. PMID: 30053207 Free PMC article.
-
Will oncotype DX DCIS testing guide therapy? A single-institution correlation of oncotype DX DCIS results with histopathologic findings and clinical management decisions.Mod Pathol. 2018 Apr;31(4):562-568. doi: 10.1038/modpathol.2017.172. Epub 2017 Dec 15. Mod Pathol. 2018. PMID: 29243740
-
Oncotype DX Breast DCIS Score® Test: Impact on Radiotherapy Recommendations and Patient Decisional Anxiety.Clin Oncol (R Coll Radiol). 2025 Jun;42:103839. doi: 10.1016/j.clon.2025.103839. Epub 2025 Apr 4. Clin Oncol (R Coll Radiol). 2025. PMID: 40311271
Cited by
-
The Coming of Age of Breast Radiotherapy.Curr Oncol. 2023 May 19;30(5):5179-5181. doi: 10.3390/curroncol30050392. Curr Oncol. 2023. PMID: 37232850 Free PMC article.
-
Comparative Analyses of Van Nuys Prognostic Index and NCCN Guidelines in Ductal Carcinoma In Situ Treatment in a Brazilian Hospital.Life (Basel). 2025 Mar 9;15(3):432. doi: 10.3390/life15030432. Life (Basel). 2025. PMID: 40141777 Free PMC article.
-
DCIS Progression and the Tumor Microenvironment: Molecular Insights and Prognostic Challenges.Cancers (Basel). 2025 Jun 10;17(12):1925. doi: 10.3390/cancers17121925. Cancers (Basel). 2025. PMID: 40563575 Free PMC article. Review.
-
BRCA1 and BRCA2 Mutations in Polish Women with Ductal Carcinoma In Situ.Cancers (Basel). 2025 Feb 11;17(4):613. doi: 10.3390/cancers17040613. Cancers (Basel). 2025. PMID: 40002208 Free PMC article.
-
Tools to Guide Radiation Oncologists in the Management of DCIS.Healthcare (Basel). 2024 Apr 6;12(7):795. doi: 10.3390/healthcare12070795. Healthcare (Basel). 2024. PMID: 38610216 Free PMC article. Review.
References
-
- Ballard-Barbash R., Taplin S.H., Yankaskas B.C., Ernster V.L., Rosenberg R.D., Carney P.A., Barlow W.E., Geller B.M., Kerlikowske K., Edwards B.K., et al. Breast Cancer Surveillance Consortium: A national mammography screening and outcomes database. AJR Am. J. Roentgenol. 1997;169:1001–1008. doi: 10.2214/ajr.169.4.9308451. - DOI - PubMed
-
- Ernster V.L., Ballard-Barbash R., Barlow W.E., Zheng Y., Weaver D.L., Cutter G., Yankaskas B.C., Rosenberg R., Carney P.A., Kerlikowske K., et al. Detection of Ductal Carcinoma In Situ in Women Undergoing Screening Mammography. J. Natl. Cancer Inst. 2002;94:1546–1554. doi: 10.1093/jnci/94.20.1546. - DOI - PubMed
-
- NCCN Clinical Practice Guidelines Breast. Version 5. 2020. [(accessed on 1 April 2023)]. Available online: https://www2.tri-kobe.org/nccn/guideline/breast/english/breast.pdf.
-
- Olivotto I., Levine M., Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer Clinical practice guidelines for the care and treatment of breast cancer: The management of ductal carcinoma in situ (summary of the 2001 update) CMAJ Can. Med. Assoc. J. 2001;165:912–913. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

