Development of a Redox-Polymer-Based Electrochemical Glucose Biosensor Suitable for Integration in Microfluidic 3D Cell Culture Systems
- PMID: 37366947
- PMCID: PMC10295976
- DOI: 10.3390/bios13060582
Development of a Redox-Polymer-Based Electrochemical Glucose Biosensor Suitable for Integration in Microfluidic 3D Cell Culture Systems
Abstract
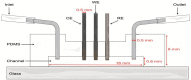
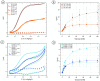
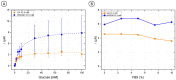
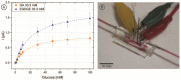
The inclusion of online, in situ biosensors in microfluidic cell cultures is important to monitor and characterize a physiologically mimicking environment. This work presents the performance of second-generation electrochemical enzymatic biosensors to detect glucose in cell culture media. Glutaraldehyde and ethylene glycol diglycidyl ether (EGDGE) were tested as cross-linkers to immobilize glucose oxidase and an osmium-modified redox polymer on the surface of carbon electrodes. Tests employing screen printed electrodes showed adequate performance in a Roswell Park Memorial Institute (RPMI-1640) media spiked with fetal bovine serum (FBS). Comparable first-generation sensors were shown to be heavily affected by complex biological media. This difference is explained in terms of the respective charge transfer mechanisms. Under the tested conditions, electron hopping between Os redox centers was less vulnerable than H2O2 diffusion to biofouling by the substances present in the cell culture matrix. By employing pencil leads as electrodes, the incorporation of these electrodes in a polydimethylsiloxane (PDMS) microfluidic channel was achieved simply and at a low cost. Under flow conditions, electrodes fabricated using EGDGE presented the best performance with a limit of detection of 0.5 mM, a linear range up to 10 mM, and a sensitivity of 4.69 μA mM-1 cm-2.
Keywords: 3D cell culture; EGDGE; cross-linking; glucose electrochemical biosensor; glucose oxidase; glutaraldehyde; on-chip evaluation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ahmed T. Organ-on-a-chip microengineering for bio-mimicking disease models and revolutionizing drug discovery. Biosens. Bioelectron. X. 2022;11:100194. doi: 10.1016/j.biosx.2022.100194. - DOI
-
- Santbergen M.J.C., van der Zande M., Bouwmeester H., Nielen M.W.F. Online and in situ analysis of organs-on-a-chip. TrAC Trends Anal. Chem. 2019;115:138–146. doi: 10.1016/j.trac.2019.04.006. - DOI
-
- Bavli D., Prill S., Ezra E., Levy G., Cohen M., Vinken M., Vanfleteren J., Jaeger M., Nahmias Y. Real-time monitoring of metabolic function in liver-onchip microdevices tracks the dynamics of Mitochondrial dysfunction. Proc. Natl. Acad. Sci. USA. 2016;113:E2231–E2240. doi: 10.1073/pnas.1522556113. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

