Discriminating Glioblastoma from Peritumoral Tissue by a Nanohole Array-Based Optical and Label-Free Biosensor
- PMID: 37366956
- PMCID: PMC10296434
- DOI: 10.3390/bios13060591
Discriminating Glioblastoma from Peritumoral Tissue by a Nanohole Array-Based Optical and Label-Free Biosensor
Abstract
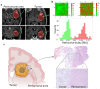
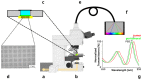
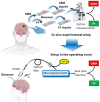
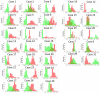
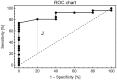
In glioblastoma (GBM) patients, maximal safe resection remains a challenge today due to its invasiveness and diffuse parenchymal infiltration. In this context, plasmonic biosensors could potentially help to discriminate tumor tissue from peritumoral parenchyma based on differences in their optical properties. A nanostructured gold biosensor was used ex vivo to identify tumor tissue in a prospective series of 35 GBM patients who underwent surgical treatment. For each patient, two paired samples, tumor and peritumoral tissue, were extracted. Then, the imprint left by each sample on the surface of the biosensor was individually analyzed, obtaining the difference between their refractive indices. The tumor and non-tumor origins of each tissue were assessed by histopathological analysis. The refractive index (RI) values obtained by analyzing the imprint of the tissue were significantly lower (p = 0.0047) in the peritumoral samples (1.341, Interquartile Range (IQR) 1.339-1.349) compared with the tumor samples (1.350, IQR 1.344-1.363). The ROC (receiver operating characteristic) curve showed the capacity of the biosensor to discriminate between both tissues (area under the curve, 0.8779, p < 0.0001). The Youden index provided an optimal RI cut-off point of 0.003. The sensitivity and specificity of the biosensor were 81% and 80%, respectively. Overall, the plasmonic-based nanostructured biosensor is a label-free system with the potential to be used for real-time intraoperative discrimination between tumor and peritumoral tissue in patients with GBM.
Keywords: biosensor; extraordinary optical transmission; glioblastoma; refractive index.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Differentiation between Glioblastoma Multiforme and Primary Cerebral Lymphoma: Additional Benefits of Quantitative Diffusion-Weighted MR Imaging.PLoS One. 2016 Sep 15;11(9):e0162565. doi: 10.1371/journal.pone.0162565. eCollection 2016. PLoS One. 2016. PMID: 27631626 Free PMC article.
-
Diagnostic value of peritumoral minimum apparent diffusion coefficient for differentiation of glioblastoma multiforme from solitary metastatic lesions.AJR Am J Roentgenol. 2011 Jan;196(1):71-6. doi: 10.2214/AJR.10.4752. AJR Am J Roentgenol. 2011. PMID: 21178049
-
The Potential Role of Peritumoral Apparent Diffusion Coefficient Evaluation in Differentiating Glioblastoma and Solitary Metastatic Lesions of the Brain.Curr Med Imaging. 2021;17(10):1200-1208. doi: 10.2174/1573405617666210316120314. Curr Med Imaging. 2021. PMID: 33726654
-
Defining optimal cutoff value of MGMT promoter methylation by ROC analysis for clinical setting in glioblastoma patients.J Neurooncol. 2017 May;133(1):193-201. doi: 10.1007/s11060-017-2433-9. Epub 2017 May 17. J Neurooncol. 2017. PMID: 28516344
-
Peritumoral tissue (PTT): increasing need for naming convention.Br J Cancer. 2024 Oct;131(7):1111-1115. doi: 10.1038/s41416-024-02828-y. Epub 2024 Sep 2. Br J Cancer. 2024. PMID: 39223304 Free PMC article. Review.
Cited by
-
Transformative biomedical devices to overcome biomatrix effects.Biosens Bioelectron. 2025 Jul 1;279:117373. doi: 10.1016/j.bios.2025.117373. Epub 2025 Mar 12. Biosens Bioelectron. 2025. PMID: 40120290 Review.
-
Malignant Cells Beyond the Tumor Core: The Non-Negligible Factor to Overcome the Refractory of Glioblastoma.CNS Neurosci Ther. 2025 Mar;31(3):e70333. doi: 10.1111/cns.70333. CNS Neurosci Ther. 2025. PMID: 40104956 Free PMC article. Review.
-
Biosensor-Enhanced Organ-on-a-Chip Models for Investigating Glioblastoma Tumor Microenvironment Dynamics.Sensors (Basel). 2024 Apr 30;24(9):2865. doi: 10.3390/s24092865. Sensors (Basel). 2024. PMID: 38732975 Free PMC article. Review.
-
Advances in Diagnostic Tools and Therapeutic Approaches for Gliomas: A Comprehensive Review.Sensors (Basel). 2023 Dec 15;23(24):9842. doi: 10.3390/s23249842. Sensors (Basel). 2023. PMID: 38139688 Free PMC article. Review.
References
-
- Tejada Solís S., Plans Ahicart G., Iglesias Lozano I., de Quintana Schmidt C., Fernández Coello A., Hostalot Panisello C., Ley Urzaiz L., García Romero J.C., Díez Valle R., González Sánchez J., et al. Glioblastoma Treatment Guidelines: Consensus by the Spanish Society of Neurosurgery Tumor Section. Neurocirugia. 2020;31:289–298. doi: 10.1016/j.neucir.2020.06.001. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

