Recent Progress in Diboronic-Acid-Based Glucose Sensors
- PMID: 37366983
- PMCID: PMC10296726
- DOI: 10.3390/bios13060618
Recent Progress in Diboronic-Acid-Based Glucose Sensors
Abstract
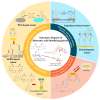
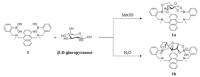
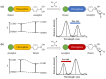
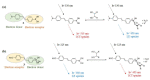
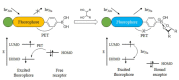
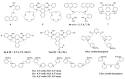
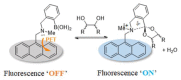
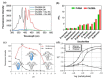
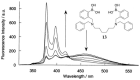
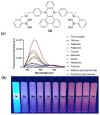
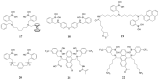
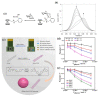
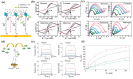
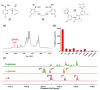
Non-enzymatic sensors with the capability of long-term stability and low cost are promising in glucose monitoring applications. Boronic acid (BA) derivatives offer a reversible and covalent binding mechanism for glucose recognition, which enables continuous glucose monitoring and responsive insulin release. To improve selectivity to glucose, a diboronic acid (DBA) structure design has been explored and has become a hot research topic for real-time glucose sensing in recent decades. This paper reviews the glucose recognition mechanism of boronic acids and discusses different glucose sensing strategies based on DBA-derivatives-based sensors reported in the past 10 years. The tunable pKa, electron-withdrawing properties, and modifiable group of phenylboronic acids were explored to develop various sensing strategies, including optical, electrochemical, and other methods. However, compared to the numerous monoboronic acid molecules and methods developed for glucose monitoring, the diversity of DBA molecules and applied sensing strategies remains limited. The challenges and opportunities are also highlighted for the future of glucose sensing strategies, which need to consider practicability, advanced medical equipment fitment, patient compliance, as well as better selectivity and tolerance to interferences.
Keywords: diboronic acid; glucose sensors; recognition mechanism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





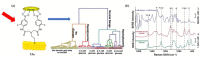
Similar articles
-
Versatile Stimuli-Responsive Controlled Release of Pinanediol-Caged Boronic Esters for Spatiotemporal and Nitroreductase-Selective Glucose Bioimaging.ACS Sens. 2025 Jan 24;10(1):470-479. doi: 10.1021/acssensors.4c02811. Epub 2025 Jan 3. ACS Sens. 2025. PMID: 39750141 Free PMC article.
-
Design and Synthesis of Novel Di-Boronic Acid-Based Chemical Glucose Sensors.ACS Omega. 2025 Mar 11;10(11):10812-10825. doi: 10.1021/acsomega.4c06237. eCollection 2025 Mar 25. ACS Omega. 2025. PMID: 40160773 Free PMC article.
-
Molecular Design of a New Diboronic Acid for the Electrohydrodynamic Monitoring of Glucose.Angew Chem Int Ed Engl. 2019 Jul 29;58(31):10612-10615. doi: 10.1002/anie.201904595. Epub 2019 Jul 1. Angew Chem Int Ed Engl. 2019. PMID: 31168957
-
Single-walled carbon nanotube-based near-infrared optical glucose sensors toward in vivo continuous glucose monitoring.J Diabetes Sci Technol. 2013 Jan 1;7(1):72-87. doi: 10.1177/193229681300700109. J Diabetes Sci Technol. 2013. PMID: 23439162 Free PMC article. Review.
-
Applications of trimetallic nanomaterials as Non-Enzymatic glucose sensors.Drug Dev Ind Pharm. 2023 Jun;49(6):393-404. doi: 10.1080/03639045.2023.2221737. Epub 2023 Jun 22. Drug Dev Ind Pharm. 2023. PMID: 37272678 Review.
Cited by
-
Versatile Stimuli-Responsive Controlled Release of Pinanediol-Caged Boronic Esters for Spatiotemporal and Nitroreductase-Selective Glucose Bioimaging.ACS Sens. 2025 Jan 24;10(1):470-479. doi: 10.1021/acssensors.4c02811. Epub 2025 Jan 3. ACS Sens. 2025. PMID: 39750141 Free PMC article.
-
Design and Synthesis of Novel Di-Boronic Acid-Based Chemical Glucose Sensors.ACS Omega. 2025 Mar 11;10(11):10812-10825. doi: 10.1021/acsomega.4c06237. eCollection 2025 Mar 25. ACS Omega. 2025. PMID: 40160773 Free PMC article.
References
-
- Ginter E., Simko V. Type 2 diabetes mellitus, pandemic in 21st century. Adv. Exp. Med. Biol. 2013;771:42–50. - PubMed
-
- Cefalu W.T., Buse J.B., Tuomilehto J., Fleming G.A., Ferrannini E., Gerstein H.C., Bennett P.H., Ramachandran A., Raz I., Rosenstock J., et al. Update and next steps for real-world translation of interventions for type 2 diabetes prevention: Reflections from a diabetes care editors’ expert forum. Diabetes Care. 2013;39:1186–1201. doi: 10.2337/dc16-0873. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

