Progress in the Optical Sensing of Cardiac Biomarkers
- PMID: 37366997
- PMCID: PMC10296523
- DOI: 10.3390/bios13060632
Progress in the Optical Sensing of Cardiac Biomarkers
Abstract
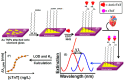
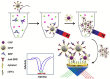
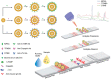
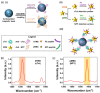
Biomarkers play key roles in the diagnosis, risk assessment, treatment and supervision of cardiovascular diseases (CVD). Optical biosensors and assays are valuable analytical tools answering the need for fast and reliable measurements of biomarker levels. This review presents a survey of recent literature with a focus on the past 5 years. The data indicate continuing trends towards multiplexed, simpler, cheaper, faster and innovative sensing while newer tendencies concern minimizing the sample volume or using alternative sampling matrices such as saliva for less invasive assays. Utilizing the enzyme-mimicking activity of nanomaterials gained ground in comparison to their more traditional roles as signaling probes, immobilization supports for biomolecules and for signal amplification. The growing use of aptamers as replacements for antibodies prompted emerging applications of DNA amplification and editing techniques. Optical biosensors and assays were tested with larger sets of clinical samples and compared with the current standard methods. The ambitious goals on the horizon for CVD testing include the discovery and determination of relevant biomarkers with the help of artificial intelligence, more stable specific recognition elements for biomarkers and fast, cheap readers and disposable tests to facilitate rapid testing at home. As the field is progressing at an impressive pace, the opportunities for biosensors in the optical sensing of CVD biomarkers remain significant.
Keywords: biosensors; cardiovascular diseases; optical assay.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
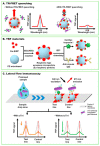
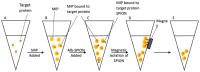
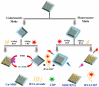


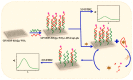
References
-
- World Health Organisation Cardiovascular Diseases. [(accessed on 8 May 2023)]; Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1.
-
- World Health Organisation Cardiovascular Diseases (CVDs) [(accessed on 8 May 2023)]; Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases...
-
- Knuuti J., Wijns W., Saraste A., Capodanno D., Barbato E., Funck-Brentano C., Prescott E., Storey R.F., Deaton C., Cuisset T., et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC) Eur. Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425. - DOI - PubMed
-
- McDonagh T.A., Metra M., Adamo M., Gardner R.S., Baumbach A., Böhm M., Burri H., Butler J., Čelutkienė J., Chioncel O., et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

