Current Practice in Using Voltage Imaging to Record Fast Neuronal Activity: Successful Examples from Invertebrate to Mammalian Studies
- PMID: 37367013
- PMCID: PMC10296598
- DOI: 10.3390/bios13060648
Current Practice in Using Voltage Imaging to Record Fast Neuronal Activity: Successful Examples from Invertebrate to Mammalian Studies
Abstract
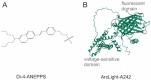
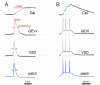
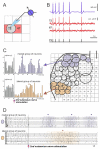
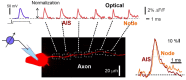
The optical imaging of neuronal activity with potentiometric probes has been credited with being able to address key questions in neuroscience via the simultaneous recording of many neurons. This technique, which was pioneered 50 years ago, has allowed researchers to study the dynamics of neural activity, from tiny subthreshold synaptic events in the axon and dendrites at the subcellular level to the fluctuation of field potentials and how they spread across large areas of the brain. Initially, synthetic voltage-sensitive dyes (VSDs) were applied directly to brain tissue via staining, but recent advances in transgenic methods now allow the expression of genetically encoded voltage indicators (GEVIs), specifically in selected neuron types. However, voltage imaging is technically difficult and limited by several methodological constraints that determine its applicability in a given type of experiment. The prevalence of this method is far from being comparable to patch clamp voltage recording or similar routine methods in neuroscience research. There are more than twice as many studies on VSDs as there are on GEVIs. As can be seen from the majority of the papers, most of them are either methodological ones or reviews. However, potentiometric imaging is able to address key questions in neuroscience by recording most or many neurons simultaneously, thus providing unique information that cannot be obtained via other methods. Different types of optical voltage indicators have their advantages and limitations, which we focus on in detail. Here, we summarize the experience of the scientific community in the application of voltage imaging and try to evaluate the contribution of this method to neuroscience research.
Keywords: GEVI; VSD; neuron; neuronal activity; optical recording; voltage imaging; voltage-sensitive dyes.
Conflict of interest statement
The authors declare no conflict of interests.
Figures
References
-
- Kuhn B., Fromherz P. Anellated Hemicyanine Dyes in a Neuron Membrane: Molecular Stark Effect and Optical Voltage Recording. J. Phys. Chem. B. 2003;107:7903–7913. doi: 10.1021/jp0345811. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

