Label-Free Electrochemical Aptasensor Based on the Vertically-Aligned Mesoporous Silica Films for Determination of Aflatoxin B1
- PMID: 37367026
- PMCID: PMC10296088
- DOI: 10.3390/bios13060661
Label-Free Electrochemical Aptasensor Based on the Vertically-Aligned Mesoporous Silica Films for Determination of Aflatoxin B1
Abstract
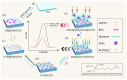
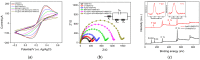
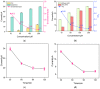
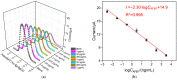
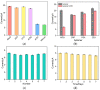
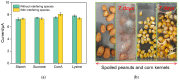
Herein we report a highly specific electrochemical aptasenseor for AFB1 determination based on AFB1-controlled diffusion of redox probe (Ru(NH3)63+) through nanochannels of AFB1-specific aptamer functionalized VMSF. A high density of silanol groups on the inner surface confers VMSF with cationic permselectivity, enabling electrostatic preconcentration of Ru(NH3)63+ and producing amplified electrochemical signals. Upon the addition of AFB1, the specific interaction between the aptamer and AFB1 occurs and generates steric hindrance effect on the access of Ru(NH3)63+, finally resulting in the reduced electrochemical responses and allowing the quantitative determination of AFB1. The proposed electrochemical aptasensor shows excellent detection performance in the range of 3 pg/mL to 3 μg/mL with a low detection limit of 2.3 pg/mL for AFB1 detection. Practical analysis of AFB1 in peanut and corn samples is also accomplished with satisfactory results by our fabricated electrochemical aptasensor.
Keywords: aflatoxin B1; electrochemical aptasensor; vertically aligned mesoporous silica films.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
A semiconductor quantum dot-based ratiometric electrochemical aptasensor for the selective and reliable determination of aflatoxin B1.Analyst. 2019 Aug 5;144(16):4772-4780. doi: 10.1039/c9an00825j. Analyst. 2019. PMID: 31268094
-
Sensitivity programmable ratiometric electrochemical aptasensor based on signal engineering for the detection of aflatoxin B1 in peanut.J Hazard Mater. 2020 Apr 5;387:122001. doi: 10.1016/j.jhazmat.2019.122001. Epub 2019 Dec 30. J Hazard Mater. 2020. PMID: 31901843
-
Vertically-Ordered Mesoporous Silica Film Based Electrochemical Aptasensor for Highly Sensitive Detection of Alpha-Fetoprotein in Human Serum.Biosensors (Basel). 2023 Jun 6;13(6):628. doi: 10.3390/bios13060628. Biosensors (Basel). 2023. PMID: 37366993 Free PMC article.
-
Recent progress in optical and electrochemical aptasensor technologies for detection of aflatoxin B1.Crit Rev Food Sci Nutr. 2024;64(33):13093-13111. doi: 10.1080/10408398.2023.2260508. Epub 2023 Oct 1. Crit Rev Food Sci Nutr. 2024. PMID: 37778392 Review.
-
Recent Advances in the Aptamer-Based Electrochemical Biosensors for Detecting Aflatoxin B1 and Its Pertinent Metabolite Aflatoxin M1.Sensors (Basel). 2020 Jun 8;20(11):3256. doi: 10.3390/s20113256. Sensors (Basel). 2020. PMID: 32521629 Free PMC article. Review.
Cited by
-
Highly sensitive electrochemical immunosensor based on electrodeposited platinum nanostructures confined in silica nanochannels for the detection of the carcinoembryonic antigen.Front Chem. 2023 Oct 20;11:1271556. doi: 10.3389/fchem.2023.1271556. eCollection 2023. Front Chem. 2023. PMID: 37927568 Free PMC article.
-
A highly sensitive immunosensor based on nanochannel-confined nano-gold enhanced electrochemiluminescence for procalcitonin detection.Front Chem. 2023 Oct 9;11:1274424. doi: 10.3389/fchem.2023.1274424. eCollection 2023. Front Chem. 2023. PMID: 37876852 Free PMC article.
-
Carbon Nitride Nanosheets as an Adhesive Layer for Stable Growth of Vertically-Ordered Mesoporous Silica Film on a Glassy Carbon Electrode and Their Application for CA15-3 Immunosensor.Molecules. 2024 Sep 12;29(18):4334. doi: 10.3390/molecules29184334. Molecules. 2024. PMID: 39339328 Free PMC article.
-
Sensitive Electrochemical Detection of Carcinoembryonic Antigen Based on Biofunctionalized Nanochannel Modified Carbonaceous Electrode.Molecules. 2024 Feb 15;29(4):858. doi: 10.3390/molecules29040858. Molecules. 2024. PMID: 38398610 Free PMC article.
-
Vertically ordered mesoporous silica film-assisted electrochemical cytosensor for the sensitive detection of HeLa cells.Front Chem. 2023 Sep 1;11:1222067. doi: 10.3389/fchem.2023.1222067. eCollection 2023. Front Chem. 2023. PMID: 37727833 Free PMC article.
References
-
- Gacem M.A., Ould El Hadj-Khelil A. Toxicology, biosynthesis, bio-control of aflatoxin and new methods of detection. Asian Pac. J. Trop. Biomed. 2016;6:808–814. doi: 10.1016/j.apjtb.2016.07.012. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

