Metabolic Silencing via Methionine-Based Amino Acid Restriction in Head and Neck Cancer
- PMID: 37367038
- PMCID: PMC10297726
- DOI: 10.3390/cimb45060289
Metabolic Silencing via Methionine-Based Amino Acid Restriction in Head and Neck Cancer
Abstract
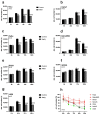
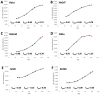
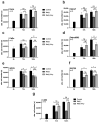
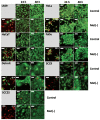
In recent years, various forms of caloric restriction (CR) and amino acid or protein restriction (AAR or PR) have shown not only success in preventing age-associated diseases, such as type II diabetes and cardiovascular diseases, but also potential for cancer therapy. These strategies not only reprogram metabolism to low-energy metabolism (LEM), which is disadvantageous for neoplastic cells, but also significantly inhibit proliferation. Head and neck squamous cell carcinoma (HNSCC) is one of the most common tumour types, with over 600,000 new cases diagnosed annually worldwide. With a 5-year survival rate of approximately 55%, the poor prognosis has not improved despite extensive research and new adjuvant therapies. Therefore, for the first time, we analysed the potential of methionine restriction (MetR) in selected HNSCC cell lines. We investigated the influence of MetR on cell proliferation and vitality, the compensation for MetR by homocysteine, the gene regulation of different amino acid transporters, and the influence of cisplatin on cell proliferation in different HNSCC cell lines.
Keywords: HNSCC; SCCHN; SLC-family; amino acid restriction; amino acid transporter; caloric restriction; cell vitality; cisplatin; low energy metabolism; methionine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Cysteine Restriction in Murine L929 Fibroblasts as an Alternative Strategy to Methionine Restriction in Cancer Therapy.Int J Mol Sci. 2021 Oct 27;22(21):11630. doi: 10.3390/ijms222111630. Int J Mol Sci. 2021. PMID: 34769059 Free PMC article.
-
Metabolic Fingerprinting of Murine L929 Fibroblasts as a Cell-Based Tumour Suppressor Model System for Methionine Restriction.Int J Mol Sci. 2021 Mar 16;22(6):3039. doi: 10.3390/ijms22063039. Int J Mol Sci. 2021. PMID: 33809777 Free PMC article.
-
Low Energy Status under Methionine Restriction Is Essentially Independent of Proliferation or Cell Contact Inhibition.Cells. 2022 Feb 4;11(3):551. doi: 10.3390/cells11030551. Cells. 2022. PMID: 35159360 Free PMC article.
-
Role of Vitamin D in Head and Neck Cancer-Immune Function, Anti-Tumour Effect, and Its Impact on Patient Prognosis.Nutrients. 2023 May 31;15(11):2592. doi: 10.3390/nu15112592. Nutrients. 2023. PMID: 37299554 Free PMC article. Review.
-
Targeting Signalling Cross-Talk between Cancer Cells and Cancer-Associated Fibroblast through Monocarboxylate Transporters in Head and Neck Cancer.Anticancer Agents Med Chem. 2021;21(11):1369-1378. doi: 10.2174/1871520620666200721135230. Anticancer Agents Med Chem. 2021. PMID: 32698754 Review.
Cited by
-
Advanced Molecular Solutions for Cancer Therapy-The Good, the Bad, and the Ugly of the Biomarker Paradigm.Curr Issues Mol Biol. 2024 Feb 22;46(3):1694-1699. doi: 10.3390/cimb46030109. Curr Issues Mol Biol. 2024. PMID: 38534725 Free PMC article.
References
-
- Hosios A.M., Hecht V.C., Danai L.V., Johnson M.O., Rathmell J.C., Steinhauser M.L., Manalis S.R., Vander Heiden M.G. Amino acids rather than glucose account for the majority of cell mass in proliferating mammalian cells. Dev. Cell. 2016;36:540–549. doi: 10.1016/j.devcel.2016.02.012. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

