Acceleration of the Deamination of Cytosine through Photo-Crosslinking
- PMID: 37367047
- PMCID: PMC10297672
- DOI: 10.3390/cimb45060298
Acceleration of the Deamination of Cytosine through Photo-Crosslinking
Abstract
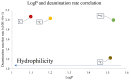
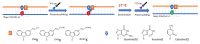
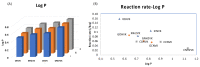
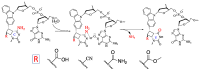
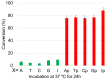
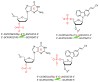
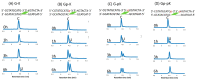
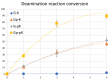
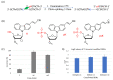
Herein, we report the major factor for deamination reaction rate acceleration, i.e., hydrophilicity, by using various 5-substituted target cytosines and by carrying out deamination at high temperatures. Through substitution of the groups at the 5'-position of the cytosine, the effect of hydrophilicity was understood. It was then used to compare the various modifications of the photo-cross-linkable moiety as well as the effect of the counter base of the cytosine to edit both DNA and RNA. Furthermore, we were able to achieve cytosine deamination at 37 °C with a half-life in the order of a few hours.
Keywords: DNA manipulation; RNA editing; cytosine deamination; nucleobase editing; photo-crosslinking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
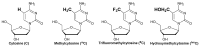



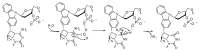
Similar articles
-
Effect of nucleobase change on cytosine deamination through DNA photo-cross-linking reaction via 3-cyanovinylcarbazole nucleoside.Mol Biosyst. 2017 Jun 1;13(6):1152-1156. doi: 10.1039/c7mb00082k. Epub 2017 Apr 28. Mol Biosyst. 2017. PMID: 28453010
-
Ultra-acceleration of Photochemical Cytosine Deamination by Using a 5'-Phosphate-Substituted Oligodeoxyribonucleotide Probe Containing a 3-Cyanovinylcarbazole Nucleotide at Its 5'-End.Chembiochem. 2018 Nov 2;19(21):2257-2261. doi: 10.1002/cbic.201800384. Epub 2018 Oct 15. Chembiochem. 2018. PMID: 30195263
-
Photochemical RNA Editing of C to U by Using Ultrafast Reversible RNA Photo-crosslinking in DNA/RNA Duplexes.Chembiochem. 2020 Nov 2;21(21):3067-3070. doi: 10.1002/cbic.202000269. Epub 2020 Jul 13. Chembiochem. 2020. PMID: 32519413
-
Control of gene expression by base deamination: the case of RNA editing in wheat mitochondria.Biochimie. 1996;78(6):511-7. doi: 10.1016/0300-9084(96)84757-2. Biochimie. 1996. PMID: 8915540 Review.
-
Mutagenic mechanisms in leukemia and cancer: a new concept Cytosine lack could be as mutagenic as cytosine deamination.Leuk Res. 2006 Sep;30(9):1079-83. doi: 10.1016/j.leukres.2005.12.019. Epub 2006 Jul 3. Leuk Res. 2006. PMID: 16820204 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

