Large-Scale Production of Anti-RNase A VHH Expressed in pyrG Auxotrophic Aspergillus oryzae
- PMID: 37367053
- PMCID: PMC10297652
- DOI: 10.3390/cimb45060304
Large-Scale Production of Anti-RNase A VHH Expressed in pyrG Auxotrophic Aspergillus oryzae
Abstract
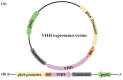
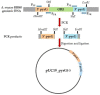
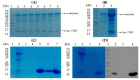
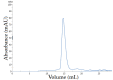
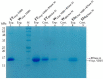
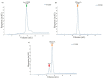
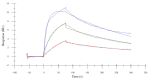
Nanobodies, also referred to as VHH antibodies, are the smallest fragments of naturally produced camelid antibodies and are ideal affinity reagents due to their remarkable properties. They are considered an alternative to monoclonal antibodies (mAbs) with potential utility in imaging, diagnostic, and other biotechnological applications given the difficulties associated with mAb expression. Aspergillus oryzae (A. oryzae) is a potential system for the large-scale expression and production of functional VHH antibodies that can be used to meet the demand for affinity reagents. In this study, anti-RNase A VHH was expressed under the control of the glucoamylase promoter in pyrG auxotrophic A. oryzae grown in a fermenter. The feature of pyrG auxotrophy, selected for the construction of a stable and efficient platform, was established using homologous recombination. Pull-down assay, size exclusion chromatography, and surface plasmon resonance were used to confirm the binding specificity of anti-RNase A VHH to RNase A. The affinity of anti-RNase A VHH was nearly 18.3-fold higher (1.9 nM) when expressed in pyrG auxotrophic A. oryzae rather than in Escherichia coli. This demonstrates that pyrG auxotrophic A. oryzae is a practical, industrially scalable, and promising biotechnological platform for the large-scale production of functional VHH antibodies with high binding activity.
Keywords: Aspergillus oryzae; VHH; affinity reagent; nanobodies; pyrG; single domain antibodies; surface plasmon resonance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
A new and efficient approach for construction of uridine/uracil auxotrophic mutants in the filamentous fungus Aspergillus oryzae using Agrobacterium tumefaciens-mediated transformation.World J Microbiol Biotechnol. 2017 Jun;33(6):107. doi: 10.1007/s11274-017-2275-9. Epub 2017 May 2. World J Microbiol Biotechnol. 2017. PMID: 28466303
-
The construction and use of versatile binary vectors carrying pyrG auxotrophic marker and fluorescent reporter genes for Agrobacterium-mediated transformation of Aspergillus oryzae.World J Microbiol Biotechnol. 2016 Dec;32(12):204. doi: 10.1007/s11274-016-2168-3. Epub 2016 Nov 1. World J Microbiol Biotechnol. 2016. PMID: 27804102
-
Development of a new Agrobacterium-mediated transformation system based on a dual auxotrophic approach in the filamentous fungus Aspergillus oryzae.World J Microbiol Biotechnol. 2021 May 4;37(6):92. doi: 10.1007/s11274-021-03060-z. World J Microbiol Biotechnol. 2021. PMID: 33945073
-
Small Antibodies with Big Applications: Nanobody-Based Cancer Diagnostics and Therapeutics.Cancers (Basel). 2023 Nov 29;15(23):5639. doi: 10.3390/cancers15235639. Cancers (Basel). 2023. PMID: 38067344 Free PMC article. Review.
-
Single domain camel antibodies: current status.J Biotechnol. 2001 Jun;74(4):277-302. doi: 10.1016/s1389-0352(01)00021-6. J Biotechnol. 2001. PMID: 11526908 Review.
Cited by
-
Aspergillus oryzae as a Cell Factory: Research and Applications in Industrial Production.J Fungi (Basel). 2024 Mar 26;10(4):248. doi: 10.3390/jof10040248. J Fungi (Basel). 2024. PMID: 38667919 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

