Adenovirus as a Vector and Oncolytic Virus
- PMID: 37367056
- PMCID: PMC10297569
- DOI: 10.3390/cimb45060307
Adenovirus as a Vector and Oncolytic Virus
Abstract
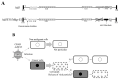
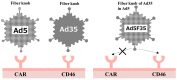
Adenoviral vectors, both oncolytic viruses and gene delivery vectors, are among the earliest approved and commercialised vectors for gene therapy. Adenoviruses have high cytotoxicity and immunogenicity. Therefore, lentiviruses or adeno-associated viruses as viral vectors and herpes simplex virus as an oncolytic virus have recently drawn attention. Thus, adenoviral vectors are often considered relatively obsolete. However, their high cargo limit and transduction efficiency are significant advantages over newer viral vectors. This review provides an overview of the new-generation adenoviral vectors. In addition, we describe the modification of the fiber knob region that enhances affinity of adenoviral vectors for cancer cells and the utilisation of cancer-cell-specific promoters to suppress expression of unwanted transgenes in non-malignant tissues.
Keywords: adenoviral vector; adenovirus; midkine; oncolytic virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Treatment of malignant gliomas with a replicating adenoviral vector expressing herpes simplex virus-thymidine kinase.Cancer Res. 2001 Dec 15;61(24):8743-50. Cancer Res. 2001. PMID: 11751394
-
Enhanced antitumor efficacy of fiber-modified, midkine promoter-regulated oncolytic adenovirus in human malignant mesothelioma.Cancer Sci. 2013 Nov;104(11):1433-9. doi: 10.1111/cas.12267. Epub 2013 Sep 23. Cancer Sci. 2013. PMID: 23962292 Free PMC article.
-
Trans-complementing adenoviral vectors for oncolytic therapy of malignant melanoma.J Gene Med. 2004 Jun;6(6):652-62. doi: 10.1002/jgm.551. J Gene Med. 2004. PMID: 15170736
-
Recent advances in genetic modification of adenovirus vectors for cancer treatment.Cancer Sci. 2017 May;108(5):831-837. doi: 10.1111/cas.13228. Epub 2017 May 7. Cancer Sci. 2017. PMID: 28266780 Free PMC article. Review.
-
Concepts in Oncolytic Adenovirus Therapy.Int J Mol Sci. 2021 Sep 29;22(19):10522. doi: 10.3390/ijms221910522. Int J Mol Sci. 2021. PMID: 34638863 Free PMC article. Review.
Cited by
-
Clinical Advances and Future Directions of Oncolytic Virotherapy for Head and Neck Cancer.Cancers (Basel). 2023 Nov 4;15(21):5291. doi: 10.3390/cancers15215291. Cancers (Basel). 2023. PMID: 37958464 Free PMC article. Review.
-
Current Landscape of Gene Therapy for the Treatment of Cardiovascular Disorders.Curr Gene Ther. 2024;24(5):356-376. doi: 10.2174/0115665232268840231222035423. Curr Gene Ther. 2024. PMID: 38288826 Review.
-
Strategies for Modifying Adenoviral Vectors for Gene Therapy.Int J Mol Sci. 2024 Nov 20;25(22):12461. doi: 10.3390/ijms252212461. Int J Mol Sci. 2024. PMID: 39596526 Free PMC article. Review.
-
Clinical and Translational Landscape of Viral Gene Therapies.Cells. 2024 Nov 19;13(22):1916. doi: 10.3390/cells13221916. Cells. 2024. PMID: 39594663 Free PMC article. Review.
-
Gene therapy of adeno-associated virus (AAV) vectors in preclinical models of ischemic stroke.CNS Neurosci Ther. 2023 Dec;29(12):3725-3740. doi: 10.1111/cns.14392. Epub 2023 Aug 8. CNS Neurosci Ther. 2023. PMID: 37551863 Free PMC article. Review.
References
-
- Gene Therapy Clinical Trials Worldwide. 2022. [(accessed on 28 February 2023)]. Available online: https://a873679.fmphost.com/fmi/webd/GTCT.
-
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

