Magnolia kobus Extract Suppresses Porphyromonas gingivalis LPS-Induced Proinflammatory Cytokine and MMP Expression in HGF-1 Cells and Regulates Osteoclastogenesis in RANKL-Stimulated RAW264.7 Cells
- PMID: 37367059
- PMCID: PMC10297525
- DOI: 10.3390/cimb45060310
Magnolia kobus Extract Suppresses Porphyromonas gingivalis LPS-Induced Proinflammatory Cytokine and MMP Expression in HGF-1 Cells and Regulates Osteoclastogenesis in RANKL-Stimulated RAW264.7 Cells
Abstract
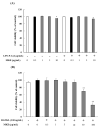
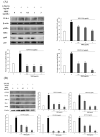
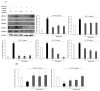
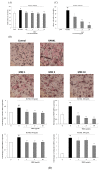
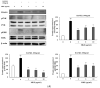
Clinical prevention is of utmost importance for the management of periodontal diseases. Periodontal disease starts with an inflammatory response in the gingival tissue, and results in alveolar bone destruction and subsequent tooth loss. This study aimed to confirm the anti-periodontitis effects of MKE. To confirm this, we studied its mechanism of action using qPCR and WB in LPS-treated HGF-1 cells and RANKL-induced osteoclasts. We found that MKE suppressed proinflammatory cytokine protein expression by inhibiting the TLR4/NF-κB pathway in LPS-PG-induced HGF-1 cells and blocking ECM degradation by regulating the expression of TIMPs and MMPs. We also confirmed that TRAP activity and multinucleated cell formation were reduced in RANKL-stimulated osteoclasts after exposure to MKE. These results were confirmed by inhibiting TRAF6/MAPK expression, which led to the suppression of NFATc1, CTSK, TRAP, and MMP expression at the gene and protein levels. Our results confirmed that MKE is a promising candidate for the management of periodontal disease based on its anti-inflammatory effects and inhibition of ECM degradation and osteoclastogenesis.
Keywords: ECM degradation; Magnolia kobus; bone resorption; gingival tissue destruction; human gingival fibroblast; inflammation; osteoclast; periodontitis.
Conflict of interest statement
Author H.J.L., S.J.L., S.K.L., B.K.C., D.R.L. was employed by the company NUON Co. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Magnolia kobus Extract Inhibits Periodontitis-Inducing Mediators in Porphyromonas gingivalis Lipopolysaccharide-Activated RAW 264.7 Cells.Curr Issues Mol Biol. 2023 Jan 6;45(1):538-554. doi: 10.3390/cimb45010036. Curr Issues Mol Biol. 2023. PMID: 36661522 Free PMC article.
-
Myricetin suppresses LPS-induced MMP expression in human gingival fibroblasts and inhibits osteoclastogenesis by downregulating NFATc1 in RANKL-induced RAW 264.7 cells.Arch Oral Biol. 2012 Dec;57(12):1623-32. doi: 10.1016/j.archoralbio.2012.06.012. Epub 2012 Jul 12. Arch Oral Biol. 2012. PMID: 22795564
-
Silibinin alleviates inflammation-induced bone loss by modulating biological interaction between human gingival fibroblasts and monocytes.J Periodontol. 2023 Jul;94(7):905-918. doi: 10.1002/JPER.22-0535. Epub 2023 Mar 8. J Periodontol. 2023. PMID: 36716169
-
Recombinant thrombomodulin lectin-like domain attenuates Porphyromonas gingivalis lipopolysaccharide-induced osteoclastogenesis and periodontal bone resorption.J Periodontol. 2021 Nov;92(11):1622-1634. doi: 10.1002/JPER.20-0732. Epub 2021 Feb 6. J Periodontol. 2021. PMID: 33438207
-
Immune response: the key to bone resorption in periodontal disease.J Periodontol. 2005 Nov;76(11 Suppl):2033-41. doi: 10.1902/jop.2005.76.11-S.2033. J Periodontol. 2005. PMID: 16277573 Review.
Cited by
-
Magnolia kobus DC. Alleviates adenine-induced chronic kidney disease by regulating ferroptosis in C57BL/6 mice.Front Pharmacol. 2025 Apr 29;16:1548660. doi: 10.3389/fphar.2025.1548660. eCollection 2025. Front Pharmacol. 2025. PMID: 40365315 Free PMC article.
References
-
- Herath T.D., Wang Y., Seneviratne C.J., Darveau R.P., Wang C.-Y., Jin L. The expression and regulation of matrix metalloproteinase-3 is critically modulated by Porphyromonas gingivalis lipopolysaccharide with heterogeneous lipid A structures in human gingival fibroblasts. BMC Microbiol. 2013;13:73. doi: 10.1186/1471-2180-13-73. - DOI - PMC - PubMed
-
- Yoon H.-S., Kim H.J., Park C.-M. Anti-inflammatory activity of various seaweeds in LPS-PG stimulated HGF-1 cells. J. Korean Soc. Oral Health Sci. 2020;8:25–30. doi: 10.33615/jkohs.2020.8.4.25. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

