Repurposing of the Drug Tezosentan for Cancer Therapy
- PMID: 37367074
- PMCID: PMC10297528
- DOI: 10.3390/cimb45060325
Repurposing of the Drug Tezosentan for Cancer Therapy
Abstract
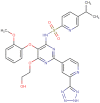
Tezosentan is a vasodilator drug that was originally developed to treat pulmonary arterial hypertension. It acts by inhibiting endothelin (ET) receptors, which are overexpressed in many types of cancer cells. Endothelin-1 (ET1) is a substance produced by the body that causes blood vessels to narrow. Tezosentan has affinity for both ETA and ETB receptors. By blocking the effects of ET1, tezosentan can help to dilate blood vessels, improve the blood flow, and reduce the workload on the heart. Tezosentan has been found to have anticancer properties due to its ability to target the ET receptors, which are involved in promoting cellular processes such as proliferation, survival, neovascularization, immune cell response, and drug resistance. This review intends to demonstrate the potential of this drug in the field of oncology. Drug repurposing can be an excellent way to improve the known profiles of first-line drugs and to solve several resistance problems of these same antineoplastic drugs.
Keywords: cancer; drug repurposing; endothelin receptors; tezosentan.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Pulmonary vasoconstrictor influence of endothelin in exercising swine depends critically on phosphodiesterase 5 activity.Am J Physiol Lung Cell Mol Physiol. 2014 Mar 1;306(5):L442-52. doi: 10.1152/ajplung.00057.2013. Epub 2014 Jan 10. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 24414253 Free PMC article.
-
Tezosentan, a combined parenteral endothelin receptor antagonist, produces pulmonary vasodilation in lambs with acute and chronic pulmonary hypertension.Pediatr Crit Care Med. 2004 Nov;5(6):571-7. doi: 10.1097/01.PCC.0000137357.52609.F0. Pediatr Crit Care Med. 2004. PMID: 15530195
-
Hemodynamic effects of tezosentan, an intravenous dual endothelin receptor antagonist, in patients with class III to IV congestive heart failure.Circulation. 2001 Feb 20;103(7):973-80. doi: 10.1161/01.cir.103.7.973. Circulation. 2001. PMID: 11181472 Clinical Trial.
-
Endothelin in the pulmonary circulation with special reference to hypoxic pulmonary vasoconstriction.Scand Cardiovasc J Suppl. 1997;46:1-40. Scand Cardiovasc J Suppl. 1997. PMID: 9265559 Review.
-
The endothelin system in pulmonary arterial hypertension.Cardiovasc Res. 2004 Feb 1;61(2):227-37. doi: 10.1016/j.cardiores.2003.11.026. Cardiovasc Res. 2004. PMID: 14736539 Review.
Cited by
-
Enhancing Urological Cancer Treatment: Leveraging Vasodilator Synergistic Potential with 5-FU for Improved Therapeutic Outcomes.J Clin Med. 2024 Jul 14;13(14):4113. doi: 10.3390/jcm13144113. J Clin Med. 2024. PMID: 39064153 Free PMC article.
-
Drug Repurposing for Cancer Treatment: A Comprehensive Review.Int J Mol Sci. 2024 Nov 19;25(22):12441. doi: 10.3390/ijms252212441. Int J Mol Sci. 2024. PMID: 39596504 Free PMC article. Review.
-
The Potential of Dutasteride for Treating Multidrug-Resistant Candida auris Infection.Pharmaceutics. 2024 Jun 14;16(6):810. doi: 10.3390/pharmaceutics16060810. Pharmaceutics. 2024. PMID: 38931930 Free PMC article.
-
Understanding the Clinical Use of Levosimendan and Perspectives on its Future in Oncology.Biomolecules. 2023 Aug 24;13(9):1296. doi: 10.3390/biom13091296. Biomolecules. 2023. PMID: 37759695 Free PMC article. Review.
References
-
- Alfarouk K.O., Stock C.M., Taylor S., Walsh M., Muddathir A.K., Verduzco D., Bashir A.H., Mohammed O.Y., Elhassan G.O., Harguindey S., et al. Resistance to cancer chemotherapy: Failure in drug response from ADME to P-gp. Cancer Cell. Int. 2015;15:71. doi: 10.1186/s12935-015-0221-1. - DOI - PMC - PubMed
-
- Khataniar A., Pathak U., Rajkhowa S., Jha A.N. A Comprehensive Review of Drug Repurposing Strategies against Known Drug Targets of COVID-19. COVID. 2022;2:148–167. doi: 10.3390/covid2020011. - DOI
Publication types
LinkOut - more resources
Full Text Sources

