Para-Methoxybenzylidene Acetal-Protected D-Glucosamine Derivatives as pH-Responsive Gelators and Their Applications for Drug Delivery
- PMID: 37367116
- PMCID: PMC10298149
- DOI: 10.3390/gels9060445
Para-Methoxybenzylidene Acetal-Protected D-Glucosamine Derivatives as pH-Responsive Gelators and Their Applications for Drug Delivery
Abstract
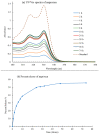
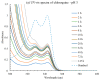
Carbohydrate-based low molecular weight gelators (LMWGs) are compounds with the capability to self-assemble into complex molecular networks within a solvent, leading to solvent immobilization. This process of gel formation depends on noncovalent interactions, including Van der Waals, hydrogen bonding, and π-π stacking. Due to their potential applications in environmental remediation, drug delivery, and tissue engineering, these molecules have emerged as an important area of research. In particular, various 4,6-O-benzylidene acetal-protected D-glucosamine derivatives have shown promising gelation abilities. In this study, a series of C-2-carbamate derivatives containing a para-methoxy benzylidene acetal functional group were synthesized and characterized. These compounds exhibited good gelation properties in several organic solvents and aqueous mixtures. Upon removal of the acetal functional group under acidic conditions, a number of deprotected free sugar derivatives were also synthesized. Analysis of these free sugar derivatives revealed two compounds were hydrogelators while their precursors did not form hydrogels. For those protected carbamates that are hydrogelators, removal of the 4,6-protection will result in a more water-soluble compound that produces a transition from gel to solution. Given the ability of these compounds to form gels from solution or solution from gels in situ in response to acidic environments, these compounds may have practical applications as stimuli-responsive gelators in an aqueous medium. In turn, one hydrogelator was studied for the encapsulation and release of naproxen and chloroquine. The hydrogel exhibited sustained drug release over a period of several days, with the release of chloroquine being faster at lower pH due to the acid lability of the gelator molecule. The synthesis, characterization, gelation properties, and studies on drug diffusion are discussed.
Keywords: carbohydrate; hydrogelators; organogelators; pH-responsive; self-assembly; stimuli-responsive.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

