Semi-Solid Dosage Forms Containing Pranoprofen-Loaded NLC as Topical Therapy for Local Inflammation: In Vitro, Ex Vivo and In Vivo Evaluation
- PMID: 37367119
- PMCID: PMC10298032
- DOI: 10.3390/gels9060448
Semi-Solid Dosage Forms Containing Pranoprofen-Loaded NLC as Topical Therapy for Local Inflammation: In Vitro, Ex Vivo and In Vivo Evaluation
Abstract
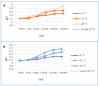
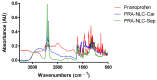
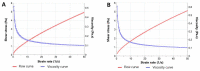
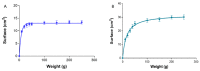
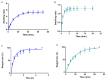
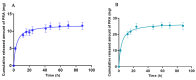
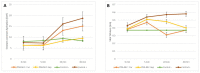
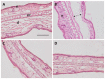
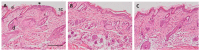
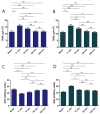
Pranoprofen (PRA)-loaded nanostructured lipid carriers (NLC) have been dispersed into blank gels composed of 1% of Carbomer 940 (PRA-NLC-Car) and 3% of Sepigel® 305 (PRA-NLC-Sep) as a novel strategy to refine the biopharmaceutical profile of PRA, for dermal administration in the treatment of skin inflammation that may be caused by possible skin abrasion. This stratagem intends to improve the joining of PRA with the skin, improving its retention and anti-inflammatory effect. Gels were evaluated for various parameters such as pH, morphology, rheology, and swelling. In vitro drug release research and ex vivo permeation through the skin were carried out on Franz diffusion cells. Additionally, in vivo assays were carried out to evaluate the anti-inflammatory effect, and tolerance studies were performed in humans by evaluating the biomechanical properties. Results showed a rheological profile common of semi-solid pharmaceutical forms for dermal application, with sustained release up to 24 h. In vivo studies using PRA-NLC-Car and PRA-NLC-Sep in Mus musculus mice and hairless rats histologically demonstrated their efficacy in an inflammatory animal model study. No signs of skin irritation or modifications of the skin's biophysical properties were identified and the gels were well tolerated. The results obtained from this investigation concluded that the developed semi-solid formulations represent a fitting drug delivery carrier for PRA's transdermal delivery, enhancing its dermal retention and suggesting that they can be utilized as an interesting and effective topical treatment for local skin inflammation caused by a possible abrasion.
Keywords: Carbomer 940; Sepigel® 305; biomechanical properties; drug delivery; inflammation; nanostructured lipid carriers; pranoprofen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Laura Guerrero Sánchez E., Fierro Arias L., María Ponce Olivera R., Peniche Castellanos A. Revisión de Técnicas de Abrasión Cutánea. Dermatol. Cosmética Médica Quirúrgica. 2015;13:66–71.
LinkOut - more resources
Full Text Sources
Research Materials

