Preparation and Characterization of Biocompatible Iron/Zirconium/Polydopamine/Carboxymethyl Chitosan Hydrogel with Fenton Catalytic Properties and Photothermal Efficacy
- PMID: 37367123
- PMCID: PMC10298290
- DOI: 10.3390/gels9060452
Preparation and Characterization of Biocompatible Iron/Zirconium/Polydopamine/Carboxymethyl Chitosan Hydrogel with Fenton Catalytic Properties and Photothermal Efficacy
Abstract
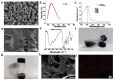
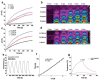
In recent years, multifunctional hydrogel nanoplatforms for the synergistic treatment of tumors have received a great deal of attention. Here, we prepared an iron/zirconium/polydopamine/carboxymethyl chitosan hydrogel with Fenton and photothermal effects, promising for future use in the field of synergistic therapy and prevention of tumor recurrence. The iron (Fe)-zirconium (Zr)@ polydopamine (PDA) nanoparticles were synthesized by a simple one-pot hydrothermal method using iron (III) chloride hexahydrate (FeCl3•6H2O), zirconium tetrachloride (ZrCl4), and dopamine, followed by activation of the carboxyl group of carboxymethyl chitosan (CMCS) using 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC)/N(4)-hydroxycytidine (NHS). Finally, the Fe-Zr@PDA nanoparticles and the activated CMCS were mixed to form a hydrogel. On the one side, Fe ions can use hydrogen peroxide (H2O2) which is rich in the tumor microenvironment (TME) to produce toxic hydroxyl radicals (•OH) and kill tumor cells, and Zr can also enhance the Fenton effect; on the other side, the excellent photothermal conversion efficiency of the incorporated PDA is used to kill tumor cells under the irradiation of near-infrared light. The ability of Fe-Zr@PDA@CMCS hydrogel to produce •OH and the ability of photothermal conversion were verified in vitro, and swelling and degradation experiments confirmed the effective release and good degradation of this hydrogel in an acidic environment. The multifunctional hydrogel is biologically safe at both cellular and animal levels. Therefore, this hydrogel has a wide range of applications in the synergistic treatment of tumors and the prevention of recurrence.
Keywords: Fenton reaction; chemodynamic therapy; hydrogel; photothermal therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Frei E., III Curative cancer chemotherapy. Cancer Res. 1985;45:6523–6537. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

