Conceptualization and Investigation of Multicomponent Polymer Networks as Prospective Corticosteroid Carriers
- PMID: 37367141
- PMCID: PMC10298209
- DOI: 10.3390/gels9060470
Conceptualization and Investigation of Multicomponent Polymer Networks as Prospective Corticosteroid Carriers
Abstract
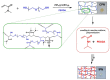
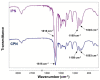
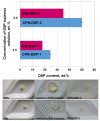
Dexamethasone (DXM) is a highly potent and long-acting synthetic glucocorticoid with anti-inflammatory, anti-allergic, and immunosuppressive effects. However, the systemic application of DXM can cause undesirable side effects: sleep disorders, nervousness, heart rhythm disorders, heart attack, and others. In the present study, multicomponent polymer networks were developed as potential new platforms for the dermal application of dexamethasone sodium phosphate (DSP). First, a copolymer network (CPN) comprising hydrophilic segments of different chemical structures was synthesized by applying redox polymerization of dimethyl acrylamide onto poly(ethylene glycol) in the presence of poly(ethylene glycol) diacrylate (PEGDA) as a crosslinker. On this basis, an interpenetrating polymer network structure (IPN) was obtained by introducing a second network of PEGDA-crosslinked poly(N-isopropylacrylamide). Multicomponent networks obtained were characterized by FTIR, TGA, and swelling kinetics in different solvents. Both CPN and IPN showed a high swelling degree in aqueous media (up to 1800 and 1200%, respectively), reaching the equilibrium swelling within 24 h. Additionally, IPN showed temperature-responsive swelling in an aqueous solution as the equilibrium swelling degree decreased considerably with an increase in the temperature. In order to evaluate the networks' potential as drug carriers, swelling in DSP aqueous solutions of varied concentration was investigated. It was established that the amount of encapsulated DSP could be easily controlled by the concentration of drug aqueous solution. In vitro DSP release was studied in buffer solution (BS) with pH 7.4 at 37 °C. The results obtained during DSP loading and release experiments proved the feasibility of the developed multicomponent hydrophilic polymer networks as effective platforms for potential dermal application.
Keywords: copolymer network; dermal application; dexamethasone sodium phosphate; drug delivery; interpenetrating network.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Yasir M., Goyal A., Sonthalia S. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2022. Corticosteroid Adverse Effects. - PubMed
-
- European Medicines Agency . Assessment Report of Dexamethasone Alapis. European Medicines Agency; Amsterdam, The Netherlands: 2011.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

