Exploring the Impact of Alginate-PVA Ratio and the Addition of Bioactive Substances on the Performance of Hybrid Hydrogel Membranes as Potential Wound Dressings
- PMID: 37367146
- PMCID: PMC10297600
- DOI: 10.3390/gels9060476
Exploring the Impact of Alginate-PVA Ratio and the Addition of Bioactive Substances on the Performance of Hybrid Hydrogel Membranes as Potential Wound Dressings
Abstract
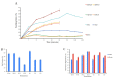
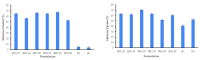
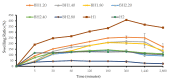
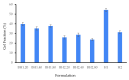
Healthcare professionals face an ongoing challenge in managing both acute and chronic wounds, given the potential impact on patients' quality of life and the limited availability of expensive treatment options. Hydrogel wound dressings offer a promising solution for effective wound care due to their affordability, ease of use, and ability to incorporate bioactive substances that enhance the wound healing process. Our study aimed to develop and evaluate hybrid hydrogel membranes enriched with bioactive components such as collagen and hyaluronic acid. We utilized both natural and synthetic polymers and employed a scalable, non-toxic, and environmentally friendly production process. We conducted extensive testing, including an in vitro assessment of moisture content, moisture uptake, swelling rate, gel fraction, biodegradation, water vapor transmission rate, protein denaturation, and protein adsorption. We evaluated the biocompatibility of the hydrogel membranes through cellular assays and performed instrumental tests using scanning electron microscopy and rheological analysis. Our findings demonstrate that the biohybrid hydrogel membranes exhibit cumulative properties with a favorable swelling ratio, optimal permeation properties, and good biocompatibility, all achieved with minimal concentrations of bioactive agents.
Keywords: bioactive; biohybrid; collagen; dressing; healing; hyaluronan; hydrogel; wound.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




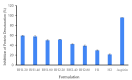



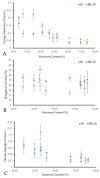
References
-
- Zhu X., Olsson M.M., Bajpai R., Järbrink K., Tang W.E., Car J. Health-Related Quality of Life and Chronic Wound Characteristics among Patients with Chronic Wounds Treated in Primary Care: A Cross-Sectional Study in Singapore. Int. Wound J. 2022;19:1121–1132. doi: 10.1111/iwj.13708. - DOI - PMC - PubMed
-
- Laurano R., Boffito M., Ciardelli G., Chiono V. Wound Dressing Products: A Translational Investigation from the Bench to the Market. Eng. Regen. 2022;3:182–200. doi: 10.1016/j.engreg.2022.04.002. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

